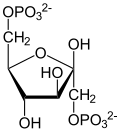
Fructose 1,6 bisphosphate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| β- D isomer | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Fructose 1,6 bisphosphate | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula | C 6 H 14 O 12 P 2 | ||||||||||||||||||
| Brief description |
colorless solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 340.12 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| solubility |
50 mg ml −1 water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Fructose-1,6-bisphosphate or fructose-1,6-biphosphate , abbreviated as F-1,6-BP, is a phosphorylated sugar that plays an important role as an intermediate in the metabolism ( glycolysis ) . The β- D form is the most common isomer found in nature . It should not be confused with fructose-2,6-bisphosphate , a very similar metabolite with regulatory activity.
history
One of the earliest scientific mentions is from 1933 studying the intermediates of glycolysis. At this point the compound was named fructose diphosphoric acid. The prefix “to” is used today because it is more correct. It indicates that the two phosphate groups are not connected to each other and are located on two different atoms.
Biological importance
Fructose-1,6-bisphosphate is an important intermediate in carbohydrate metabolism, e.g. B. in the breakdown of glucose. In the course of glycolysis, it is produced from fructose-6-phosphate , which is phosphorylated by a phosphofructokinase with consumption of ATP . In the subsequent reaction, an aldolase splits fructose-1,6-bisphosphate into two C 3 building blocks: glyceraldehyde-3-phosphate and dihydroxyacetone phosphate .
F-1,6-BP is also formed when glucose is built up (gluconeogenesis). This is then converted into fructose-6-phosphate, which is then further processed into glucose-6-phosphate .
Finally, F-1,6-BP can also regulate pyruvate kinase : The pyruvate kinases of the liver , kidneys and erythrocytes are allosterically activated by fructose-1,6-bisphosphate .
Individual evidence
- ↑ a b Data sheet D-Fructose-1-6-bisphosphate-trisodium-salt-hydrate ( Memento from March 4, 2016 in the Internet Archive ) at scbt.com, accessed May 5, 2012.
- ↑ a b Data sheet D-Fructose 1,6-bisphosphate sodium salt hydrate, ≥70% from Sigma-Aldrich , accessed on February 13, 2013 ( PDF ).
- ↑ Embden, G. et al. (1933): About the intermediate processes in glycolysis in the muscles In: Klinische Wochenschrift 12 (6), pp. 213–215; doi : 10.1007 / BF01757728
- ^ H. Robert Horton, Laurence A. Moran, K. Gray Scrimgeour, Marc D. Perry, J. David Rawn and Carsten Biele (translators): Biochemie . Pearson Studies; 4th updated edition 2008; ISBN 978-3-8273-7312-0 ; P. 469.
- ↑ Jeremy M. Berg, Lubert Stryer and John L. Tymoczko: Biochemistry . Spectrum Academic Publishing House; 6th edition 2007; ISBN 978-3-8274-1800-5 ; P. 520.