Glycolysis
The glycolysis ( , ancient Greek γλυκύς glykys , sweet 'and λύσις lysis , Resolution') is by living beings of the phasing monosaccharides (simple sugars) such as D - glucose (dextrose), from which the name of glycolysis is derived. It is the central process in the breakdown of all carbohydrates in all eukaryotes , including animals , plants and fungi . In bacteria and archaea glycolysis is also common. However, some species also use other metabolic pathways to break down glucose, for example the Entner-Doudoroff path (ED path). Glycolysis is a central process in energy metabolism and one of the few metabolic pathways that almost all organisms have in common, which indicates a very early development.
The dismantling takes place in ten individual steps. This creates two pyruvate molecules from one glucose molecule . In addition, two molecules of adenosine triphosphate (ATP) suitable for the transmission of energy are formed and two molecules of NAD + are reduced to NADH.
Glycolysis is also called Embden-Meyerhof-Parnas-Weg or EMP-Weg , after its discoverers Gustav Embden , Otto Meyerhof and Jakub Karol Parnas . The term FDP path , which goes back to the intermediate product D - fructose-1,6-bisphosphate (outdated: fructose diphosphate), is no longer in use .
Discovery story

Studies on the breakdown of sugar go back well into the 19th century and originally began with research into alcoholic fermentation and, later, lactic acid fermentation . In these fermentations, the reaction steps up to the formation of pyruvate are identical. In 1837, researchers Charles Cagniard-Latour , Theodor Schwann and Friedrich Traugott Kützing independently demonstrated that the breakdown of glucose to ethanol, known today as alcoholic fermentation , is caused by living things, namely yeasts . The fact that the metabolic processes of living yeast cells are responsible for the anaerobic breakdown of sugars was still very controversial at the time. The prominent chemists Jöns Jakob Berzelius , Friedrich Wöhler and Justus von Liebig were among the most violent opponents of this view. Liebig postulated, for example, that decaying material transfers “vibrations” to the sugar to be fermented, which then breaks down into ethanol and carbon dioxide.
From 1857, the French researcher Louis Pasteur also devoted himself to the breakdown of sugars in living yeast cells . In 1860 he published a confirmation of the results of Cagniard-Latour, Kützing and Schwann and thus opposed Liebig's hypothesis. He also observed that the consumption of glucose is higher under anaerobic conditions than when oxygen is available to the yeast. This observation is now known as the “ Pasteur effect ”.
At that time, the prevailing doctrine was that only a “ life force ” (vis vitalis) inherent in living beings could convert glucose into ethanol. In 1858, on the other hand, Moritz Traube suggested that chemical processes alone were responsible for the breakdown of sugars in yeast cells, rather than “liveliness” as such. In 1897, Eduard Buchner finally discovered that alcoholic fermentation is also possible in a cell-free yeast extract. He showed that the metabolic pathway can take place even if the cells are no longer intact. This is known as in vitro . He referred to the catalytically active preparation as “ zymase ”, without knowing that several enzymes are involved in the anaerobic breakdown of glucose. Even Marie of Mannasein moved in the same year in a publication similar conclusions as Buchner. However, their work failed to convince others because their evidence was insufficient.

The elucidation of the individual steps of glycolysis succeeded from the beginning of the 20th century. So could Arthur Harden and William John Young (1878-1942) decisive in resolving the glycolytic pathway contribute and published their findings in a series of publications from 1905. Among other things, they found that isolated yeast extracts glucose mined slowly to ethanol and carbon dioxide, when no inorganic phosphate was present in the extracts. With the addition of phosphate, however, this fermentation reaction , which took place in vitro , i.e. without living cells, could proceed more quickly. They also succeeded in isolating fructose-1,6-bisphosphate and demonstrating that it is an intermediate product of glycolysis. They also separated cell-free yeast extract into two fractions using dialysis . The researchers called the non-dialysable fraction, which are usually larger molecules and proteins, according to Buchner as “zymase”. She was sensitive to heat. The dialyzable fraction, on the other hand, consists of ions and small molecules that can pass through the dialysis membrane. This was thermally stable and was called "Cozymase". Only both together could induce a fermentation reaction in vitro . It turned out that the zymase was a mixture of enzymes, while the coenzymase contained the coenzymes necessary for these enzymes .
In 1918 Otto Meyerhof was able to prove that the same coenzymes are required in lactic acid fermentation in muscles as in alcoholic fermentation. Due to the short life of many intermediate products, further elucidation of the metabolic pathway turned out to be difficult. Gustav Embden proposed the first biochemical reaction sequence for glycolysis in 1932. Two years later, Karl Lohmann was able to prove in Meyerhof's laboratory that the universal energy carrier adenosine triphosphate (ATP) is generated during glycolysis. Meyerhof's research group contributed to the discovery of about a third of the enzymes involved in glycolysis.
Finally, at the end of the 1930s, the work of Otto Warburg and Hans von Euler-Chelpin clarified the reaction steps in yeast; Embden, Meyerhof and Jakub Karol Parnas , on the other hand, worked with muscle cells. In addition, Carl and Gerty Cori , Carl Neuberg , Robert Robinson and Erwin Negel, who worked under Warburg, played an important role in the elucidation of glycolysis.
All the steps and enzymes involved in glycolysis have been known since the 1940s. More detailed studies of the enzymes involved and their regulation then followed.
Meaning for the cell
Glycolysis is the most important way of breaking down carbohydrates in the metabolism. Most of all hexoses and Trioses be this one pathway metabolized and prepared for further degradation. Glycolysis thus occupies a central place in the catabolic metabolism . The enzymes involved in the reactions occur in almost all living things, so that glycolysis is also universal. Glycolysis also has other important functions:
Energy generation under anaerobic conditions
In glycolysis, energy is obtained and provided in the form of two molecules of ATP per molecule of degraded D- glucose, regardless of whether there is oxygen for the respiratory chain or not. Glycolysis produces about a fifteenth as much ATP per molecule of D -glucose as the complete oxidative breakdown to carbon dioxide and water in the citric acid cycle and in the respiratory chain. Therefore, less glucose is metabolized under aerobic conditions, which was already observed in yeast by Louis Pasteur in 1861 ( Pasteur effect ).
Since glycolysis also takes place under anoxic conditions, this opens up some beneficial possibilities in the metabolism. For example, microorganisms can gain energy in this way in an anoxic environment. In vertebrates , when the muscles are used to a great extent, more oxygen is sometimes consumed than is transported into the cells. Therefore, the cell has to get its energy exclusively from glycolysis for a short time. This is often the case with larger animals such as alligators , crocodiles , elephants , rhinos , whales and seals , where oxygen cannot be provided quickly enough for the oxidative breakdown of glucose. In humans, too, glucose is converted to lactate in rapidly contracting muscle cells in the course of glycolysis and lactic acid fermentation . A great advantage of glycolysis is the fact that ATP can be made available 100 times as fast as via oxidative phosphorylation in the respiratory chain.
Plants get their energy either from photosynthesis or from the respiratory chain. However, there are also situations in which light and oxygen are temporarily not available, for example during imbibition during seed germination or when the roots are temporarily flooded with water. Under these conditions, the local metabolism is maintained by glycolysis.
Glucose as the only fuel
Cells in the brain have to get most of their energy from glycolysis, some specialized cells even get their energy exclusively from glycolysis. This includes, for example, cells of the renal medulla , also erythrocytes , which lack mitochondria and thus the respiratory chain, and sperm . After all, it also includes rapidly growing and dividing tumor cells . Otto Warburg discovered in 1930 that tumor cells have a much higher rate of glycolysis than healthy cells. In the positron emission tomography , this is used to visualizing tumor tissue.
Building blocks for cell material
Glycolysis not only prepares glucose for oxidative degradation, but also provides precursors for the biosynthesis of other compounds. Thus pyruvate starting material for the synthesis of fatty acids and, for some amino acids ( L - alanine , L - valine and L - leucine ). Glycerol-3-phosphate is reductively formed from dihydroxyacetone phosphate , which plays a role in the synthesis of lipids . Phosphoenolpyruvate is the starting material for the biosynthesis of the aromatic amino acids L - phenylalanine , L - tryptophan and L - tyrosine , while L - serine is formed from 3-phosphoglycerate .
Provision of NADH
In addition to ATP, glycolysis also produces the reducing agent NADH. This is either reoxidized in the respiratory chain for a further gain of ATP, or used as a reducing agent for the synthesis of other molecules - at least for the purpose of NAD + regeneration in fermentations .
Reaction steps
Cellular localization
Glycolysis takes place in the cytoplasm of a cell . In multicellular organisms such as humans, glycolysis is carried out in all (differentiated) cell types. Plants also carry out glycolysis in the plastids .
General overview

Glu-6-P = glucose-6-phosphate
Fru-6-P = fructose-6-phosphate
Fru-1,6-bP = fructose-1,6-bisphosphate
DHAP = dihydroxyacetone phosphate
GAP = glyceraldehyde-3-phosphate
1 , 3-bPG = 1,3-bisphosphoglycerate
3-PG = 3-phosphoglycerate
2-PG = 2-phosphoglycerate
PEP = phosphoenolpyruvate
Pyr = pyruvate
The breakdown of glucose to pyruvate takes place in the same way both under oxygen deficiency conditions ( anaerobic ) and when there is sufficient oxygen supply ( aerobic ). In contrast to the respiratory chain, no oxygen (O 2 ) is consumed.
Glycolysis can be divided into two phases. The first phase is a preparatory phase in which energy is first invested in the form of ATP. It consists of the cleavage of the hexose D- glucose into two triosephosphates: dihydroxyacetone phosphate (DHAP) and glyceraldehyde-3-phosphate (GAP) (see figure). Here DHAP is in CAP for the second phase isomerization . This prepares the sugar for the actual breakdown.
In the second phase, two GAP molecules are converted into two pyruvate (Pyr) molecules via several intermediate steps. Two molecules of NADH and four molecules of ATP are formed. This phase provides energy in the form of 4 ATP and 2 reduction equivalents NADH.
The total balance of glycolysis can thus be formulated as follows:
Preparatory phase
The first step in glycolysis is the phosphorylation of D- glucose (Glc) to glucose-6-phosphate (G6P). Depending on the cell type, this reaction is a hexokinase or glucokinase (= hexokinase IV) catalyzes , in which one molecule of ATP is invested. This has two advantages: On the one hand, the cell membrane is permeable to glucose, but not to the glucose-6-phosphate produced by phosphorylation. As a result, it accumulates in the cell. On the other hand, the phosphorylation of glucose reduces the glucose concentration in the cell, while the concentration of G6P increases in reverse. The initial phosphorylation has the effect that there is less glucose inside the cell than outside the cell. Since the intracellular glucose concentration is in imbalance with the extracellular one, further glucose flows into the cell as a result of this concentration gradient. As a result, the absorption of glucose is favored.
In bacteria, the phosphorylation in the first step of glycolysis is not catalyzed by hexo- or glucokinases, but by the phosphoenolpyruvate (PEP) -dependent sugar phosphotransferase system .
Glucose-6-phosphate is then converted into the isomeric fructose-6-phosphate (F6P) by the glucose-6-phosphate isomerase . The enzyme prefers the binding of the alpha anomer of G6P, the reaction product is α- D- fructose-6-phosphate. Under standard conditions, the equilibrium of the isomerization reaction is on the side of the G6P. But since F6P continues to react quickly, it is withdrawn from the reaction system, so that no equilibrium is established and the isomerization reaction proceeds in favor of the F6P.
 |
ATP ADP hexokinase or glucokinase |
 |
Glucose- 6-phosphate isomerase  |

|
| α- D - glucose | α- D - glucose-6-phosphate | α- D - fructose-6-phosphate |
Fructose-6-phosphate is then under the action of the first key enzyme of glycolysis, phosphofructokinase 1 , phosphorylated with a molecule of ATP to fructose-1,6-bisphosphate (F1,6bP), whereby ADP is formed. The enzyme prefers the beta anomer of F6P, whereas the alpha anomer was formed in the preliminary reaction. However, this is not a problem since the two anomers are in equilibrium ( mutarotation ). In anaerobic bacteria, some plants, primitive eukaryotes and some archaea, this step is catalyzed by a pyrophosphate- dependent phosphofructokinase (EC 2.7.1.90), in which pyrophosphate (PP i , from English pyrophosphate, inorganic ) is used instead of ATP.
 |
ATP ADP phospho fructo kinase |

|
| β- D- fructose-6-phosphate | β- D - fructose-1,6-bisphosphate |
The renewed investment of energy is cheap and necessary for two reasons: On the one hand, this step - in addition to glucokinase and pyruvate kinase - makes glycolysis irreversible under physiological conditions. On the other hand, the energy supplied here enables the hexose to be split in the next step and thus the formation of two phosphorylated trioses for further degradation, dihydroxyacetone phosphate (DHAP) and glyceraldehyde-3-phosphate (GAP). The C1-C3 carbon atoms of F1,6bP are found in DHAP, while the C atoms in GAP come from the C4-C6 unit of F1,6bP.
The cleavage reaction is very unfavorable under standard conditions (ΔG 0 '= +24 kJ / mol) and would not take place. Due to the rapid metabolism of both reaction products, however, it takes place in near equilibrium under physiological conditions. Dihydroxyacetone phosphate is also converted into D- glyceraldehyde-3-phosphate by the triosephosphate isomerase (TIM) . This stereospecific isomerization in the direction of GAP is favored by the fact that GAP is further broken down in the glycolysis and thus the concentration in the cell is kept low. Without further metabolism, the balance between DHAP and GAP would be strongly on the side of the ketone (22: 1).
 |
Aldolase |
 |
Triose phosphate isomerase  |

|
| β- D- fructose-1,6-bisphosphate | Dihydroxy acetone phosphate |
D - glycerol aldehyde 3-phosphate |
Amortization phase
Each of the two resulting glyceraldehyde-3-phosphate molecules is oxidized by a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) at the beginning of the amortization phase of glycolysis . In the reaction, NAD + is reduced to NADH. The oxidation of the aldehyde group (GAP) to the carboxy group is energetically very favorable. It is used to link inorganic phosphate with the carboxy group. This creates the mixed acid anhydride 1,3-bisphosphoglycerate (1,3-BPG). The equilibrium of this reaction is on the part of the educt GAP versus 1,3-BPG (10: 1). However, the rapid conversion of the product prevents the equilibrium from being established and 1,3-BPG is constantly formed, and a high concentration of NAD + compared to NADH favors the conversion in one direction.
An alternative by-route in erythrocytes , from 1,3-bisphosphoglycerate to 3-phosphoglycerate, is the Rapoport-Luebering cycle , which runs via the intermediate 2,3-bisphosphoglycerate , the central enzyme of which is the trifunctional bisphosphoglycerate mutase .
 |
NAD + NADH + P i + H + glyceraldehyde- 3-phosphate dehydrogenase  |

|
| D -Glyceraldehyde-3-phosphate | D - 1,3 bisphosphoglycerate |
In the next step, the phosphoglycerate kinase generates one molecule of ATP when converting 1,3-bisphosphoglycerate to 3-phosphoglycerate by transferring a phosphate residue to ADP. The energy released during the previous oxidation is thus conserved by building up ATP. The formation of ATP from ADP that takes place here is an example of substrate chain phosphorylation . If the cell already has a lot of ATP (and thus little ADP), the reaction continues at this point until enough ADP is available again. This feedback regulation is important because ATP breaks down relatively quickly if it is not used. This prevents overproduction of ATP.
The energy balance of glycolysis is balanced in this step: two molecules of ATP were used and two were recovered
 |
Phospho glycerate kinase ADP ATP 
|
|
| D -1,3-bisphosphoglycerate | D - 3-phosphoglycerate |
A cofactor-independent phosphoglycerate mutase (PGM) then catalyzes the conversion of 3-phosphoglycerate to 2-phosphoglycerate. During the process, the phosphate group is temporarily transferred to an amino acid residue of the enzyme. In erythrocytes, this reaction is catalyzed by a cofactor-dependent PGM, in which 2,3-bisphosphoglylcerate is formed as an intermediate product.
2-phosphoglycerate is then dehydrated to phosphoenolpyruvate (PEP) with the help of enolase . This is why the enzyme is also called 2-phosphoglycerate dehydratase. PEP is a phosphorylated compound with a very high group transfer potential . This is used in the last step of glycolysis to gain another molecule of ATP. Here a pyruvate kinase (PK) catalyzes the conversion of PEP to pyruvate (= anion of pyruvic acid) with ATP gain. However, this does not result in pyruvate directly, but rather the equilibrium enolpyruvate. At pH 7 the equilibrium is on the side of the ketone form. This step is also regulated by ADP; it is the third, irreversible reaction in the course of glycolysis.
 |
Phospho glycerate mutase 
|
 |
−H 2 O enolase  |
 |
ADP ATP pyruvate kinase |

|
| D -3-phosphoglycerate | D - 2-phosphoglycerate | Phosphoenolpyruvate | Pyruvate |
If there is a lack of phosphate, plants can hydrolyse PEP to pyruvate without obtaining ATP, which takes place in the vacuoles . The enzyme involved is a PEP phosphatase (EC 3.1.3.60), which releases inorganic phosphate and thus counteracts the phosphate deficiency.
Regeneration of the cofactor NAD +
In glycolysis, two molecules of NAD + are reduced to NADH per cycle. Most of the time, the cellular concentration of NAD + is very low, so that it would be used up quickly without reoxidation. As a result, NAD + has to be regenerated, otherwise glycolysis will come to a standstill. How this happens depends on whether the conditions are anoxic or oxic . In addition, this influences the further degradation path of the pyruvate.
Oxic conditions
Under oxic conditions, pyruvate is oxidatively decarboxylated in the pyruvate dehydrogenase complex to acetyl-CoA . This produces one molecule of carbon dioxide and one molecule of NADH. Acetyl-CoA then enters the citric acid cycle in which it is fully oxidized to two molecules of carbon dioxide. Additional NADH molecules are formed in these oxidation steps. These and those resulting from glycolysis are finally reoxidized in the course of the respiratory chain with the consumption of oxygen, and NAD + is thus available again for glycolysis and the citric acid cycle. At the same time, further molecules of ATP are formed during these steps. While prokaryotes can produce a total of 38 molecules of ATP per molecule of glucose, the balance in eukaryotes depends on the way in which NADH formed in the cytosol passes the mitochondrial membrane ( malate-aspartate shuttle or glycerol-3-phosphate shuttle ).
In eukaryotes, the citric acid cycle takes place in the matrix of the mitochondrion , whereas glycolysis takes place in the cytosol. NAD + and NADH cannot diffuse freely through the inner membrane of the mitochondrion, and special translocators are missing . The exchange of NAD + and NADH therefore takes place either through the malate-aspartate shuttle or the glycerol-3-phosphate shuttle.
In the literature, glycolysis and the subsequent breakdown of pyruvate to carbon dioxide through the processes of the citric acid cycle and the respiratory chain are sometimes incorrectly summarized as aerobic glycolysis . However, glycolysis ends with the formation of pyruvate and takes place under both oxic and anoxic conditions.
Anoxic conditions
→ see also main articles alcoholic fermentation , lactic acid fermentation
If oxygen is not available or only to a limited extent, pyruvate can be converted further reductively, for example in lactic acid fermentation or in alcoholic fermentation . In lactic acid fermentation, pyruvate is reduced to L - lactate with NADH , in alcoholic fermentation it is decarboxylated and reduced to ethanol . In both cases, NADH is oxidized to NAD + and is available for further rounds of glycolysis. In these fermentation steps , however, in contrast to the aerobic breakdown path, no ATP is formed.
In alcoholic fermentation, yeasts form ethanol from pyruvate in two reaction steps that are catalyzed by two enzymes, pyruvate decarboxylase (EC 4.1.1.1) and alcohol dehydrogenase . The NADH generated by glycolysis is oxidized to NAD + . Bacteria, for example lactic acid bacteria, as well as muscle cells in humans, operate the lactic acid fermentation (see also figure on the right). Here, pyruvate is reduced to lactate by lactate dehydrogenase using NADH, so that glycolysis can continue. This reaction is strongly exergonic both under standard conditions and under physiological conditions (ΔG 0 ′ = -25 kJ / mol or ΔG = -14.8 kJ / mol).
The (homofermentative) lactic acid fermentation is sometimes referred to as anaerobic glycolysis . However, this is misleading as glycolysis ends with the formation of pyruvate and takes place under both oxic and anoxic conditions.
Since glycolysis takes place in the cytosol of cells, it can also take place in cells without mitochondria. Here, however, NADH cannot be oxidized to NAD + by the citric acid cycle and the respiratory chain , but without oxygen consumption by reducing pyruvate to lactate, catalyzed by lactate dehydrogenase. Thus, when a glucose molecule is broken down, only two molecules of ATP are produced in the erythrocytes, which, however, meet the needs of these cells.
Energetic aspects
Equilibrium position
| step | Reaction in glycolysis | ΔG 0 '[kJ / mol] | ΔG [kJ / mol] |
|---|---|---|---|
| 1 | Glucose + ATP → glucose-6-P + ADP | −16.7 | −33.9 |
| 2 | Glucose-6-P ⇌ fructose-6-P | +1.7 | −2.9 |
| 3 | Fructose-6-P + ATP → fructose-1,6-bP + ADP | −14.2 | −18.8 |
| 4th | Fructose-1,6-bP ⇌ DHAP + G-3-P | +23.9 | −0.2 |
| 5 | DHAP ⇌ GAP | +7.6 | +2.4 |
| 6th | GAP + P i + NAD + ⇌ 1,3-bis-P-glycerate + NADH + H + | +6.3 | −1.3 |
| 7th | 1,3-bis-P-glycerate + ADP ⇌ 3-P-glycerate + ATP | −18.9 | +0.1 |
| 8th | 3-phosphoglycerate ⇌ 2-phosphoglycerate | +4.4 | +0.8 |
| 9 | 2-P-glycerate ⇌ PEP + H 2 O | +7.5 and +1.8, respectively | +1.1 |
| 10 | PEP + ADP → pyruvate + ATP | −31.7 | −23.0 |
Most glycolysis reactions are energetically unfavorable under standard conditions at a pH of 7. The change in the free enthalpy G 0 ' is often positive, so that those reactions are endergonic and would not take place (cf. table ΔG 0 ' values). The glycolysis would end in the fourth step.
| Metabolite | Concentration [mM] |
|---|---|
| glucose | 5.0 |
| Glucose-6-P | 0.083 |
| Fructose-6-P | 0.014 |
| Fructose-1,6-bP | 0.031 |
| DHAP | 0.140 |
| Cap | 0.019 |
| 1,3-bis-P-glycerate | 0.001 |
| 3-PG | 0.120 |
| 2-PG | 0.030 |
| PEP | 0.023 |
| Pyruvate | 0.051 |
| P i | 0.001 |
By definition, the molar concentration of the reactants corresponds to 1 mol·l −1 under such conditions . However, this cannot be used as a basis for a calculation, since living cells cannot generate or maintain such high concentrations. For a meaningful assessment, on the other hand, one would have to know the actual substance concentrations. If this is measured under physiological conditions, the change in the free enthalpy G can be recalculated (see table ΔG values, metabolite concentration).
Erythrocytes in particular are suitable for calculating these values. Erythrocytes draw all of their energy from glycolysis. All other cellular reactions also take place in the cytoplasm, since they have neither mitochondria , a cell nucleus nor an endoplasmic reticulum . This also facilitates the separation of the cell components. Without mitochondria there are no reactions of the respiratory chain or the citric acid cycle . A quantification of the coenzymes involved would otherwise be considerably more difficult. After all, the pentose phosphate pathway only takes a small part in the metabolism of erythrocytes, and there is also no protein or lipid biosynthesis. This allows the glycolytic intermediates to be easily isolated and determined.
In 1965 the substance concentrations ( steady state ) of glycolytic intermediates from human erythrocytes were determined (see table on the right). It was found that certain intermediates are present in very low concentrations. Taking these concentrations into account, the equilibrium position of the corresponding reactions changes in such a way that, under physiological conditions, the entire glycolysis is reversible except for three reactions (ΔG approximately 0 kJ · mol −1 ).
In those reactions, the molar concentration remains so low because the products generated are quickly converted and then removed from the system through irreversible reactions. These three irreversible reactions are catalyzed by the key enzymes glucokinase or hexokinase, phosphofructokinase 1 and pyruvate kinase. Due to the rapid, irreversible conversion using one of the key enzymes, the substance concentrations of the previously generated products are sufficiently reduced - the glycolysis can proceed in one direction.
When calculating the equilibrium position under physiological conditions, small but positive ΔG values result for some reactions, for example in the isomerization of 3-phosphoglycerate to 2-phosphoglycerate (ΔG = +1.1 kJ mol −1 ). Strictly speaking, these values cannot be entirely correct, since the forward reaction can only take place with negative ΔG values. However, since glycolysis takes place, it is assumed that measurement errors in determining the substance concentrations are responsible for this contradiction.
There are two consequences of having three control points. Firstly, glycolysis can be effectively regulated at those points so that it can be quickly switched on or off depending on the energy status of the cell. Secondly, the current equilibrium also enables the reverse reaction of glycolysis, gluconeogenesis . Except for three enzymes, all glycolysis enzymes are used.
Efficiency
Under standard conditions , the conversion of D- glucose into two molecules of lactate releases 183.6 kJ / mol of energy (ΔG 0 '= −183.6 kJ / mol):
61.0 kJ / mol are required to build up two molecules of ATP from two molecules of ADP and inorganic phosphate (P i ):
Since glycolysis couples these two reactions through substrate chain phosphorylation, an energy of 122.6 kJ / mol is released:
ΔG 0 '= (−183.6 + 61) kJ mol −1 = −122.6 kJ mol −1
Under standard conditions, when glucose is broken down into lactate anaerobically, 33% of the available energy is used to build up two molecules of ATP. Since under physiological conditions around 50 kJ mol −1 are required for the build-up of ATP, the energy yield is also somewhat higher, around 50%.
regulation
Glycolysis is used to provide energy, especially if the resulting pyruvate is broken down further under aerobic conditions. If, on the other hand, there is an energetically favorable state, glucose is stored and converted into other metabolites in the course of anabolism with energy consumption.
The regulation of glycolysis is therefore of crucial importance. For example, it should not run parallel to their reverse reaction, gluconeogenesis . In such a case one speaks of an " idle process ", which senselessly uses ATP and is therefore unproductive. As an exception, it is worth mentioning the heat generation in bumblebees , which generate heat through intended, counter-rotating phosphorylation and dephosphorylation of fructose-6-phosphate to fructose-1,6-bisphosphate and vice versa.
From a biochemical point of view, irreversible reactions are controlled. Under physiological conditions there are three reactions in glycolysis that are irreversible. They are catalyzed by hexo- or glucokinase, phosphofructokinase-1 and pyruvate kinase and are therefore the target of regulation.
Hexo- and glucokinase
Hexokinase is the first enzyme in glycolysis whose activity is regulated. It phosphorylates glucose to glucose-6-phosphate (G6P) while consuming ATP, but can also use other hexoses as a substrate. G6P is the end product of the hexokinase reaction and, as such, allosterically inhibits the enzyme .
The isoenzyme of hexokinase, glucokinase, which occurs in the liver, is not inhibited by the product G6P. Unlike the hexokinase it also shows a higher K M value. Therefore, the glucokinase only replaces the hexokinase at very high glucose concentrations. Under these conditions, G6P is stored as glycogen in subsequent steps and is not broken down in glycolysis, because G6P is also diverted to other metabolic pathways. The liver therefore acts as a homeostat for the blood sugar level , as it maintains it by building up or breaking down glucose.
A liver-specific regulatory protein can reversibly bind to glucokinase and thereby inhibit it. The binding of this regulator to the glucokinase takes place in the cell nucleus , so that the inhibited glucokinase remains inactive there and cannot be influenced by other cytosolic effectors. This bond is then strengthened if the enzyme has been allosterically modified by fructose-6-phosphate. In contrast, glucose causes this liver protein to be detached. When the blood sugar concentration is high, glucose dominates so that the regulator can dissociate and glucose is phosphorylated to glucose-6-phosphate. If the blood sugar level drops too much (below 5 mmol·l −1 ), the glucokinase is inhibited - mediated by fructose-6-phosphate. Glucose is no longer phosphorylated and can leave the liver again to be available to other organs.
Finally, glucokinase is regulated at the level of transcription . The hormone insulin influences the amount of glucokinase in the liver. A metabolic disorder occurs in patients with diabetes mellitus because they cannot produce enough insulin. They have too low levels of glucokinase, do not tolerate high blood sugar levels, and have little glucokinase in the liver.
Phosphofructokinase-1
The main control point of glycolysis is the phosphorylation of Frc-6-P to Frc-1,6-bP by phosphofructokinase-1 (PFK-1). It represents the first real glycolysis-specific step and is irreversible under physiological conditions. The enzyme has two binding sites for ATP. In addition to a high-affinity substrate binding site, the PFK-1 also has a regulatory binding site. Thus, ATP can serve both as a substrate and allosterically inhibit PFK-1. If the ATP concentrations are sufficiently high, the K M value of the enzyme is increased. This lowers the activity of the PFK-1, so that glycolysis is reduced. Nevertheless, the ATP concentrations in a cell fluctuate only slightly, so that ATP alone would not be sufficient for precise regulation. Therefore, the activity of the PFK-1 also depends on the AMP concentration and reflects the energetic supply of the cell. AMP acts as an allosteric, non-covalent activator. If the energy state of the cell is high (ATP concentration high, AMP concentration low), the enzyme is inhibited, otherwise the activity of the PFK-1 is increased to produce more ATP.
Even citrate inhibits the PFK-1 allosteric. Citrate is a key metabolite of the citric acid cycle , the primary purpose of which is to produce energy under aerobic conditions. Alternatively, various precursor molecules can be taken from the citric acid cycle. If there is a lot of citrate, the citric acid cycle is saturated. Therefore, citrate inhibits the PFK-1 in the sense of an end product inhibition, so that the glycolysis feeds the citric acid cycle less strongly.
The activity of phosphofructokinase-1 is also influenced by the pH value . A low pH inhibits the enzyme and slows down glycolysis. This happens, for example, when the muscles are strained and a lot of lactic acid is produced. This lowers the pH value in the cells.
Finally, PFK-1 is allosterically activated in micromolar concentrations by β- D- fructose-2,6-bisphosphate (F-2,6-bP). F-2,6-bP promotes glycolysis while it inhibits fructose-1,6-bisphosphatase . This is the enzyme that catalyzes the reverse reaction in gluconeogenesis at this point . Under physiological conditions, the enzyme remains in a practically inactive state without F-2,6-bP. After binding of F-2,6-bP to PFK-1, the affinity of the two inhibitors ATP and citrate is also reduced.
In bacteria, fructose-2,6-bisphosphate does not occur as an activator of the PFK-1.
Pyruvate kinase
The last step in glycolysis is irreversible and is catalyzed by pyruvate kinase (PK). This is also regulated, but in contrast to the other two enzymes in a comparatively subordinate way. Fructose-1,6-bisphosphate and AMP stimulate PK, while ATP, acetyl-CoA and L - alanine inhibit it allosterically. The isoenzyme (L-form) that predominates in the liver and intestines can, in contrast to the M-form that occurs in muscles, also be phosphorylated by protein kinase A. The activity of protein kinase A is hormonally mediated by glucagon . In phosphorylated form, this isoenzyme is then inhibited comparatively more strongly by ATP and alanine than the unmodified PK. This is supposed to slow down the breakdown of glucose in the liver so that it is more readily available for other organs. The dephosphorylation is catalyzed by a phosphatase .
Inhibitors
The enolase is inhibited by fluoride . Iodine acetate inhibits glyceraldehyde-3-phosphate dehydrogenase, which oxidizes glyceraldehyde-3-phosphate with an inorganic phosphate and with the participation of NAD + to form 1,3-bisphosphoglycerate. It modifies an SH group of the enzyme, so this inhibition can be lifted again by adding mercaptans .
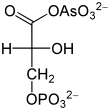
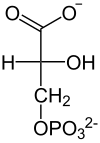
Arsenate , HAsO 4 2− , is similar to inorganic phosphate HPO 4 2− and is converted in its place by glyceraldehyde-3-phosphate dehydrogenase. This creates 1-arseno-3-phosphoglycerate from glyceraldehyde-3-phosphate (see figure). In contrast to 1,3-bisphosphoglycerate, this arsenate compound is, like any other acyl arsenate, very unstable, it breaks down to 3-phosphoglycerate. As a result, the energy of oxidation can no longer be harnessed by substrate chain phosphorylation. There is no longer a step in the formation of ATP in glycolysis, which continues to take place, but no longer supplies any net ATP. Since arsenate is not consumed, it already works in catalytic amounts. Harden and Young demonstrated the effect of arsenate on glycolysis at the beginning of the 20th century using yeast extracts.
pathology
Due to the central role of glycolysis in the metabolism, defects manifest themselves relatively rarely, because the affected cells die in most cases. Therefore, only a few diseases and mutations in glycolysis are known.
The best known are defects in pyruvate kinase that lead to hemolytic anemia . If the pyruvate kinase fails, no ATP is produced in the last step of glycolysis, so that blood cells cannot provide enough energy to operate ion pumps in the cytoplasmic membrane . This causes the red blood cells to swell and burst. If the activity of the triose phosphate isomerase is reduced by a mutation, this leads to the so-called triose phosphate isomerase deficiency . It leads to neurological damage and also to hemolytic anemia, often also to death. A deficiency in phosphofructokinase leads to Tarui's disease , which results in exercise -dependent muscle weakness with muscle pain and hemolytic anemia.
Entry of other metabolites
In addition to D- glucose, other metabolites can also occur in glycolysis, provided that they can be converted into one of the intermediate products occurring therein. Pentoses and tetroses are usually converted to glyceraldehyde-3-phosphate or fructose-6-phosphate by the pentose phosphate route and can then be converted further.
The degradation pathways of the di- or polysaccharides also lead to glycolysis. For example, sucrose is split into glucose and fructose. How fructose is further converted is described below. When lactose is broken down , D- glucose and D - galactose are produced , the latter is also converted into glucose and is broken down in glycolysis.
Individual monosaccharides are formed from multiple sugars through enzymatic reactions , which, if necessary after isomerization to glucose-6-phosphate or fructose-6-phosphate, can flow directly into the glycolytic breakdown pathway. A well-known example is the storage substance glycogen . A glycogen phosphorylase turns it into glucose- 1-phosphate , which is then isomerized to glucose-6-phosphate.
Glycerin
Glycerine is produced when triglycerides are broken down and can serve as a precursor to glycolysis or gluconeogenesis. A cytosolic glycerol kinase phosphorylates glycerol while consuming ATP to glycerol-3-phosphate , which is then oxidized to dihydroxyacetone phosphate. This is either catalyzed by a cytosolic glycerol-3-phosphate dehydrogenase (cGDH) or an isoenzyme in the membrane in the mitochondrion (mGDH). With the former, NAD + is reduced , with the latter, ubiquinone .
Fructose
When the disaccharide sucrose is broken down, glucose and fructose are released. In higher animals, fructose is phosphorylated to fructose-1-phosphate in the liver , which is catalyzed by a fructokinase ( ketohexokinase , FC) with consumption of ATP. Then aldolase B (fructose-1-phosphate aldolase, ALD-B) splits fructose-1-phosphate into dihydroxyacetone phosphate (DHAP) and glyceraldehyde . DHAP and glyceraldehyde are both converted to glyceraldehyde-3-phosphate. These two conversions are catalyzed by a triose phosphate isomerase (TPI) or a triose kinase (TK) described above with consumption of ATP. If fructokinase is missing, this leads to fructosuria , an autosomal recessive hereditary disease, in higher animals .
In other organs, the hexokinase can also take over the function of the fructokinase in order to phosphorylate fructose. Their affinity for glucose compared to fructose is however much higher (95% glucose, 5% fructose).
Mannose
D - Mannose is a component of various glycoproteins and polysaccharides . In order to be able to enter the glycolysis, mannose is first phosphorylated by a hexokinase to mannose-6-phosphate with consumption of ATP. This is finally isomerized to fructose-6-phosphate , which is catalyzed by mannose-6-phosphate isomerase (also phosphomannose isomerase , EC 5.3.1.8).
Sorbitol
Sorbitol can be oxidized to glucose or fructose in the polyol route .
Galactose
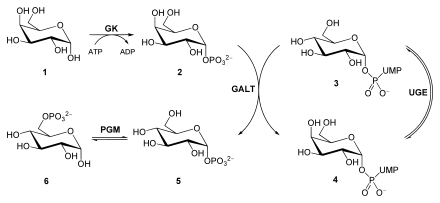
After the lactose is broken down , D- glucose and D- galactose are released. To convert galactose into its C4 epimer glucose, the sugar is first converted to galactose-1-phosphate by a galactokinase (GK) using ATP . A galactose-1-phosphate uridyltransferase (GALT) now catalyzes an exchange of UDP- bound glucose with galactose. This creates glucose-1-phosphate and UDP-galactose . While glucose-1-phosphate is isomerized to glucose-6 -phosphate by a phosphoglucomutase (PGM), a UDP-glucose-4-epimerase (UGE) epimerizes UDP-galactose to UDP-glucose .
A defect in galactokinase manifests itself in the metabolic disease galactosemia .
Special features of green plants
In green plants there are some variations in glycolysis compared to that in animals. These are described below.
Glycolysis in the plastids
It is known that in plants glycolysis is carried out independently of one another not only in the cytoplasm, but also in the plastids of the cell. However, it does not always run completely there, since enzymes in the amortization phase are often missing, for example enolase or phosphoglycerate mutase. Highly specific translocators can transport intermediates from one cell compartment to another in order to complete all glycolysis reaction steps. In the cytosol of many unicellular green algae , the cytosolic enzymes for glycolysis are missing, so that this takes place completely in the chloroplasts.
Green plants use glycolysis in the plastids to break down starch into pyruvate in the dark or in non-photosynthetic tissue with a gain in ATP and NADH. They also provide various precursor molecules for building other products, for example for fatty acid synthesis.
Isoenzymes are required for the parallel operation of glycolysis both in the cytoplasm and in the plastids . For example, there is a pyruvate kinase that is located in the cytoplasm and one that catalyzes the analogous reaction in the plastid. All isoenzymes are encoded in the genome of the plant. The plastid representatives are translated in the cytoplasm of the plant cell and then transported into the organelle . It is still unclear whether the isoenzymes resulted from gene duplication from a precursor gene . A horizontal gene transfer from the genome of a prokaryotic symbiont would also be possible ( endosymbiont theory ).
Role of pyrophosphate
Another special feature is the use of pyrophosphate (PP i ) instead of ATP as a phosphate donor in the first glycolytic reactions. This has also been observed in some bacteria. Normally, pyrophosphate is hydrolyzed to two molecules of phosphate by a pyrophosphatase (PPiase, EC 3.6.1.1). The purpose of this hydrolysis is to make biochemical reactions irreversible under physiological conditions. One speaks colloquially that this hydrolysis “pulls” the reaction to one side. The explanation for this is that the hydrolysis is exergonic, i.e. energy is released in the process:
The PPiase does not occur in the cytosol of the plants, so that a pyrophosphate concentration of up to 0.3 mmol / l can arise there. The pyrophosphate-dependent phosphofructokinase (PFP, EC 2.7.1.90) discovered in 1979 uses pyrophosphate for the phosphorylation of fructose-6-phosphate to fructose-1,6-bisphosphate. This reaction is also reversible and could also be used for the reverse route, gluconeogenesis. Like PFK-1, PFP is regulated by fructose-2,6-bisphosphate.
Metabolic diversity in phosphoenolpyruvate
Plants use PEP from glycolysis in different ways (see figure on the right). In the classic, glycolytic degradation route, the use of PEPs is limited: A pyruvate kinase (PK) uses PEP as a substrate for the direct formation of pyruvate. ATP is generated by substrate chain phosphorylation. In plants, moreover, PEP also serves as a substrate for PEP carboxykinase (PEPC), which in particular in plants with C 4 - or Crassulacean acid metabolism is important. Oxaloacetate , an intermediate of the citric acid cycle, is formed from PEP and hydrogen carbonate . Oxaloacetate is then reduced to L - malate by a cytosolic malate dehydrogenase (MDH) . L -Malat is then transported to various organelles. In the mitochondrion it can be decarboxylated to pyruvate by a mitochondrial, NAD + -dependent malate enzyme (ME). This is a bypass reaction of pyruvate kinase, in which no ATP is produced, but is useful in the case of a phosphate deficiency. This is because the phosphate bound in PEP is released again and is available to the plant for other reactions. This bypass has been demonstrated in the roots of the pea .
A plastid, NADP-dependent malate enzyme can produce pyruvate from L malate produced by PEPC and MDH . Pyruvate is required there for fatty acid synthesis or is transported back into the cytosol. This also bypasses the pyruvate kinase reaction, which is advantageous if there is a lack of phosphate.
If there is a lack of phosphate, a PEP phosphatase (PEPase, EC 3.1.3.60) can also release valuable phosphate. Here, PEP is transported into the vacuole , where it is hydrolyzed to pyruvate by a PEPase. Pyruvate and phosphate are then transported back into the cytoplasm. If there is no phosphate deficiency, the PEPase is inhibited by a sufficiently high P i concentration.
Finally, there is also a cytosolic, phosphate-independent GADH (EC 1.2.1.9) that oxidizes glyceraldehyde directly to 3-phosphoglycerate. This only produces NADPH, but no ATP.
regulation
There are some important differences between the regulation of the enzymes involved in glycolysis and that in animals. PEP is a special allosteric effector that, in contrast to the process in animals, can inhibit PFK. In contrast, fructose-1,6-bisphosphate cannot activate pyruvate kinase. While fructose-2,6-bisphosphate activates the PFK in animals, nothing like this happens in plants.
Thus, glycolysis in green plants is primarily regulated by the activities of pyruvate kinase and PEP carboxykinase, and secondly by PFK-1 and PFP. In principle, it is the other way around with animals.
Glycolysis in archaea
In the sugar-degrading archaea, carbohydrates are broken down in different ways. In hyperthermophilic and thermophilic aerobes, for example Thermoplasma acidophilum or Sulfolobus solfataricus , glucose is converted into pyruvate via a variant of the Entner-Doudoroff route (ED route). In contrast, hyperthermophilic, fermenting anaerobes such as Pyrococcus furiosus , Desulfurococcus amylolyticus , Pyrobaculum aerophilum (a microaerobe ), the sulfate reducer Archaeoglobus fulgidus and members of the Thermococcus genus use carbohydrates in a modified EMP route.
The metabolites occurring in it are similar to those of the glycolysis of eukaryotes and bacteria, but enzymes are used for this which have no similarities with those of bacteria or eukaryotes. For example, ADP- instead of ATP-dependent kinases are found in archaea, for example glucokinase (EC 2.7.1.147) or phosphofructokinase (EC 2.7.1.146). In contrast to bacteria, they do not have a PEP-dependent sugar transport system. The last step in glycolysis, the conversion of PEP to pyruvate, can also be carried out by a pyruvate phosphate dikinase (PPDK, EC 2.7.9.1) in addition to PK. This enzyme catalyzes the reversible conversion of PEP, AMP and PP i to pyruvate, ATP and P i , although the formation of pyruvate is preferred in Thermoproteus tenax . T. tenax is an anaerobically living, facultative, heterotrophic Archeon of the department Crenarchaeota . PPDK has also been detected in bacteria and eukaryotes.
One important difference is the oxidation of glyceraldehyde-3-phosphate. This is either oxidized directly to 3-phosphoglycerate by an NAD (P) + -dependent (GAPN) or a ferredoxin -dependent dehydrogenase (GAPOR), but without incorporating inorganic phosphate. Therefore, in this step, no ATP is formed through substrate chain phosphorylation, so that in most archaea no ATP is formally obtained through this modified glycolysis. The best studied glycolysis metabolic pathway in archaea is that of P. furiosus . There the net response is:
Another important difference is the lack of allosteric regulation of the key enzymes with the effectors, which was described above for bacteria and eukaryotes. In T. tenax there are at least indications that the GAPN is allosterically activated by AMP, glucose-1-phosphate, fructose-6-phosphate, fructose-1-phosphate, ADP and ribose-5-phosphate, while NAD (P) H, NADP + and ATP inhibit the enzyme. Regulation at the transcription level may also take place, as in the GAPDH found in T. tenax .
evolution
Glycolysis is found in most bacteria and eukaryotes, and in a somewhat modified form in archaea and hyperthermophilic bacteria. This suggests that glycolysis was established very early in evolution and was already present in the first organisms. By phylogenetic comparisons with thermophilic and hyperthermophilic microorganisms is suspected that the EMP pathway after the ED pathway has emerged. In addition, the original meaning of the EMP pathway was probably not in the breakdown of carbohydrates, but rather it ran the other way around as gluconeogenesis to build up glucose. This also supports the theory that metabolic pathways for building carbohydrates appeared earlier in evolution than those that break down carbohydrates; "Today's" gluconeogenesis is more widespread in organisms in all three domains than glycolysis.
The enzymes of the lower branch of glycolysis (amortization phase) mostly catalyze reactions that are reversible and are most highly conserved. They are also found in the phylogenetically older ED pathway. Like other metabolic pathways, they were probably already present before the separation of the three domains of living beings and are therefore among the oldest enzymes. Because of their essential importance, they could neither be lost nor replaced by other enzymes over time. Glyceraldehyde-3-phosphate dehydrogenase is the most highly conserved of all glycolytic enzymes; only 3% of the catalytic domain have changed in 100 million years.
The upper branch of glycolysis (preparatory phase) probably established itself later. While the enzymes involved in bacteria and eukaryotes show high homologies, the enzymes found in archaea are unique. It is still under discussion whether enzymes for the first part of glycolysis were originally lost in archaea and were only reintroduced later through horizontal gene transfer . Alternatively, enzymes with similar functions could have been used for the original glycolysis, which were then subject to major modifications and sequence changes. In either case, this could explain why the glycolytic enzymes in archaea are so different from those in other organisms.
literature
- Geoffrey Zubay: biochemistry. Mcgraw-Hill Professional. 4th edition. 1999, ISBN 3-89028-701-8 , p. 293ff.
- Donald Voet, Judith G. Voet: Biochemistry. Wiley-VCH 1994, ISBN 3-527-29249-7 , pp. 420ff.
- Jeremy M. Berg, John L. Tymoczko, Lubert Stryer: Biochemistry. 6 edition. Spectrum Akademischer Verlag, Heidelberg 2007, ISBN 978-3-8274-1800-5 , p. 486ff.
- H. Robert Horton, Laurence A. Moran, K. Gray Scrimgeour, Marc D. Perry, J. David Rawn, Carsten Biele (translator): Biochemie. 4th updated edition. Pearson Studium, 2008, ISBN 978-3-8273-7312-0 , pp. 442ff.
- Reginald Garrett, Charles M. Grisham: Biochemistry. (International Student Edition). 4th edition. Cengage Learning Services, 2009, ISBN 978-0-495-11464-2 , pp. 535-562.
- David L. Nelson, Michael M. Cox, Albert L. Lehninger (first): Lehninger Biochemie. 4th, completely revised u. exp. Edition. Springer, Berlin 2009, ISBN 978-3-540-68637-8 , pp. 607-730.
- Ron S. Ronimus, Hugh W. Morgan: Distribution and phylogenies of enzymes of the Embden-Meyerhof-Parnas pathway from archaea and hyperthermophilic bacteria support a gluconeogenic origin of metabolism. In: Archaea 1 (3) 2003, pp. 199–221, PMID 15803666 , PMC 2685568 (free full text)
- WC Plaxton: The organization and regulation of plant glycolysis. In: Annu Rev Plant Physiol Plant Mol Biol. 47 (1996), pp. 185-214, PMID 15012287 , doi : 10.1146 / annurev.arplant.47.1.185
- T. Dandekar, S. Schuster, B. Snel, M. Huynen, P. Bork: Pathway alignment: application to the comparative analysis of glycolytic enzymes . In: Biochem. J. . 343 Pt 1, October 1999, pp. 115-24. PMID 10493919 . PMC 1220531 (free full text).
Individual evidence
- ↑ C. Cagniard-Latour: Mémoire sur la fermentation vineuse. In: Annales de chimie et de physique . Vol. 68, 1837, pp. 206-222.
- ↑ Theodor Schwann: Preliminary communication, concerning experiments on fermentation and putrefaction. In: Annals of Physics and Chemistry . Vol. 41, 1837, pp. 184-193.
- ↑ FT Kützing: Microscopic investigations on yeast and mother vinegar, along with several other associated vegetable structures. In: Journ. Practical Chem . 11 (1837), pp. 385-409.
- ^ E. Racker: History of the Pasteur effect and its pathobiology. In: Mol Cell Biochem. 5 (1-2) 1974, pp. 17-23, PMID 4279327 , doi : 10.1007 / BF01874168 .
- ↑ J. Liebig: About the phenomena of fermentation, putrefaction and putrefaction and their causes. In: Annals of Pharmacy. (Heidelberg) 30 (1839), pp. 250-287.
- ^ L. Pasteur: Memoire sur la fermentaçion alcoolique. In: Annales de chimie et de physique. 58 (1860), pp. 323-426.
- ↑ M. Traube In: Ann. Phys. Chem. (Poggendorff) 103 (1858), pp. 331-344.
- ↑ E. Buchner: Alcoholic fermentation without yeast cells (preliminary communication). In: Ber. German Chem. Ges . 30 (1897), pp. 117-124. (on-line)
- ↑ Athel Cornish-Bowden: New beer bottle in an old Eduard Buchner and the growth of biochemical knowledge. ISBN 84-370-3328-4 , p. 60.
- ↑ A. Harden, WJ Young: The Alcoholic Ferment of Yeast Juice. In: Proc. R. Soc. Lond. B 77 (519) 1906, pp. 405-420, doi : 10.1098 / rspb.1906.0029 .
- ↑ A. Harden, WJ Young: The Alcoholic Ferment of Yeast Juice. Part III.-The Function of Phosphates in the Fermentation of Glucose by Yeast-Juice. In: Proc. R. Soc. Lond. B 80 (540) 1908, pp. 405-420, doi : 10.1098 / rspb.1908.0029 .
- ↑ Donald Voet, Judith G. Voet, Alfred Maelicke (ed.), Werner Müller-Esterl (ed.): Biochemie. Wiley-VCH 1992, ISBN 3-527-28242-4 , pp. 421f.
- ^ N. Kresge, RD Simoni, RL Hill: Otto Fritz Meyerhof and the elucidation of the glycolytic pathway. In: J Biol Chem . 280 (4) 2005, p. E3, PMID 15665335 , PDF (free full text access , English)
- ^ Albert L. Lehninger, David L. Nelson, Michael M. Cox: Lehninger Biochemie. 3rd, completely revised u. exp. Edition. Springer, Berlin 2009, ISBN 978-3-540-41813-9 , pp. 584ff.
- ↑ Donald Voet, Judith G. Voet, Alfred Maelicke (ed.), Werner Müller-Esterl (ed.): Biochemie. Wiley-VCH, 1992, ISBN 3-527-28242-4 , p. 444.
- ↑ Hans W. Heldt, Birgit Piechulla: Plant biochemistry . 4th edition. Spektrum Akademischer Verlag, Heidelberg 2008, ISBN 978-3-8274-1961-3 , p. 334.
- ^ Reginald Garrett, Charles M. Grisham: Biochemistry. (International Student Edition). 4th edition. Cengage Learning Services, 2009, ISBN 978-0-495-11464-2 , p. 535.
- ↑ Hans W. Heldt, Birgit Piechulla: Plant biochemistry . 4th edition. Spektrum Akademischer Verlag, Heidelberg 2008, ISBN 978-3-8274-1961-3 , p. 334.
- ↑ Jeremy M. Berg, John L. Tymoczko, Lubert Stryer: Biochemistry. 6 edition. Spektrum Akademischer Verlag, Heidelberg 2007, ISBN 978-3-8274-1800-5 , p. 764.
- ↑ Caroline Bowsher, Martin W. Steer, Alyson K. Tobin: Plant Biochemistry. Garland Pub, New York, NY 2008, ISBN 978-0-8153-4121-5 , p. 145.
- ^ H. Robert Horton, Laurence A. Moran, K. Gray Scrimgeour, Marc D. Perry, J. David Rawn, Carsten Biele (translator): Biochemie. Pearson study. 4th updated edition. 2008, ISBN 978-3-8273-7312-0 , p. 448.
- ↑ a b c R. S. Ronimus, HW Morgan: Distribution and phylogenies of enzymes of the Embden-Meyerhof-Parnas pathway from archaea and hyperthermophilic bacteria support a gluconeogenic origin of metabolism. In: Archaea . 1 (3) 2003, pp. 199-221, PMID 15803666 , PMC 2685568 (free full text).
- ^ A b Caroline Bowsher, Martin W. Steer, Alyson K. Tobin: Plant Biochemistry. Garland Pub, New York, NY 2008, ISBN 978-0-8153-4121-5 , p. 148.
- ^ David Nelson, Michael Cox: Lehninger Biochemie. Springer, Berlin; 4th, completely revised u. exp. Edition. 2008, ISBN 978-3-540-68637-8 , p. 713.
- ↑ Caroline Bowsher, Martin W. Steer, Alyson K. Tobin: Plant Biochemistry. Garland Pub, New York, NY 2008, ISBN 978-0-8153-4121-5 , p. 150.
- ^ A b c d Reginald Garrett, Charles M. Grisham: Biochemistry. (International Student Edition). 4th edition. 2009, Cengage Learning Services, ISBN 978-0-495-11464-2 , p. 538.
- ↑ a b David L. Nelson, Michael M. Cox: Lehninger Principles of Biochemistry. 5th edition. Palgrave Macmillan, 2008, ISBN 978-0-7167-7108-1 , p. 553.
- ^ Reginald Garrett, Charles M. Grisham: Biochemistry. (International Student Edition). 4th edition. Cengage Learning Services, Australia 2009, ISBN 978-0-495-11464-2 , p. 539.
- ^ S. Minakami, H. Yoshikawa: Studies on erythrocyte glycolysis. II. Free energy changes and rate limitings steps in erythrocyte glycolysis. In: J. Biochem. Vol. 59 (2) 1966, pp. 139-144, PMID 4223318 .
- ↑ S. Minakami, H. Yoshikawa: Thermodynamic considerations on erythrocyte glycolysis. In: Biochem. Biophys. Res. Commun. Vol. 18 (3) 1965, pp. 345-349, PMID 14300746 , doi : 10.1016 / 0006-291X (65) 90711-4 .
- ^ Reginald Garrett, Charles M. Grisham: Biochemistry. (International Student Edition). 4th edition. Cengage Learning Services, Australia 2009, ISBN 978-0-495-11464-2 , p. 554.
- ↑ Athel Cornish-Bowden: New beer bottle in an old Eduard Buchner and the growth of biochemical knowledge. Universitat de Valencia, Valencia 1997, ISBN 84-370-3328-4 , p. 148.
- ^ Reginald Garrett, Charles M. Grisham: Biochemistry. (International Student Edition). 4th edition. 2009, Cengage Learning Services, ISBN 978-0-495-11464-2 , p. 537.
- ^ EA Newsholme et al .: The activities of fructose diphosphatase in flight muscles from the bumble-bee and the role of this enzyme in heat generation. In: Biochem J . 128 (1) 1972, pp. 89-97, PMID 4343671 , PMC 1173573 (free full text).
- ^ A b Reginald Garrett, Charles M. Grisham: Biochemistry. (International Student Edition). Cengage learning services; 4th edition. 2009, ISBN 978-0-495-11464-2 , pp. 539f.
- ^ A b c David Nelson, Michael Cox: Lehninger Biochemie. 4th, completely revised u. exp. Edition. Springer, Berlin 2008, ISBN 978-3-540-68637-8 , pp. 773ff.
- ^ Reginald Garrett, Charles M. Grisham: Biochemistry. (International Student Edition). Cengage Learning Services. 4th edition. 2009, ISBN 978-0-495-11464-2 , pp. 542f.
- ↑ Müller-Esterl, Werner .: Biochemistry: an introduction for physicians and natural scientists . 1st edition Elsevier, Spektrum, Akad. Verl, Munich 2004, ISBN 3-8274-0534-3 , pp. 496 f .
- ^ A b c H. Robert Horton, Laurence A. Moran, K. Gray Scrimgeour, Marc D. Perry, J. David Rawn, Carsten Biele (translator): Biochemie. Pearson study. 4th updated edition, 2008, ISBN 978-3-8273-7312-0 , p. 468.
- ^ H. Robert Horton, Laurence A. Moran, K. Gray Scrimgeour, Marc D. Perry, J. David Rawn, Carsten Biele (translator): Biochemie. Pearson study. 4th updated edition, 2008, ISBN 978-3-8273-7312-0 , p. 470.
- ↑ Todd A. Swanson, Sandra I. Kim, Marc J. Glucksman: BRS Biochemistry, Molecular Biology, and Genetics. 5th edition. Lippincott Raven, 2010, ISBN 978-0-7817-9875-4 , p. 65.
- ↑ A. Harden, WJ Young: The Alcoholic Ferment of Yeast Juice. Part VI.-The Influence of Arsenates and Arsenites on the Fermentation of the Sugars by Yeast-Juice. In: Proc. R. Soc. Lond. B 83 (566) 1911, pp. 451-475, doi : 10.1098 / rspb.1911.0028 .
- ↑ Todd A. Swanson, Sandra I. Kim, Marc J. Glucksman: BRS Biochemistry, Molecular Biology, and Genetics. 5th edition. Lippincott Raven, 2010, ISBN 978-0-7817-9875-4 , p. 123.
- ^ A b W. C. Plaxton: The organization and regulation of plant glycolysis. In: Annu Rev Plant Physiol Plant Mol Biol. 47 (1996), pp. 185-214, PMID 15012287 , doi : 10.1146 / annurev.arplant.47.1.185 .
- ^ Rudolf K. Thauer, Kurt Jungermann, Karl Decker: Energy conservation in chemotrophic anaerobic bacteria. In: Bacteriological Reviews . Vol. 41, No. 1, 1977, p. 101.
- ^ B. Siebers, P. Schönheit: Unusual pathways and enzymes of central carbohydrate metabolism in Archaea. In: Curr Opin Microbiol . 8 (6) 2005, pp. 695-705, PMID 16256419 , doi : 10.1016 / j.mib.2005.10.014 .
- ↑ M. Zaparty et al .: The central carbohydrate metabolism of the hyperthermophilic crenarchaeote Thermoproteus tenax: pathways and insights into their regulation. In: Arch Microbiol . 190 (3) 2008, pp. 231-245, PMID 18491075 , doi : 10.1007 / s00203-008-0375-5 .
- ^ CH Verhees et al .: The unique features of glycolytic pathways in Archaea. In: Biochem J . 375 (Pt 2) 2003, pp. 231-246, PMID 12921536 , PMC 1223704 (free full text).
- ^ AH Romano, T. Conway: Evolution of carbohydrate metabolic pathways. In: Res. Microbiol. Vol. 147 (6-7), 1996, pp. 448-455, PMID 9084754 ; doi : 10.1016 / 0923-2508 (96) 83998-2
- ↑ RS Ronimus, HW Morgan: Distribution and phylogenies of enzymes of the Embden-Meyerhof-Parnas pathway from archaea and hyperthermophilic bacteria support a gluconeogenic origin of metabolism. In: Archaea 1 (3) 2003, pp. 199-221, PMID 15803666 , PMC 2685568 (free full text).
Web links
- Glycolysis on Ulrich Helmich's homepage
- Jennifer McDowall / Interpro: Protein Of The Month: Enzymes of Glycolysis. (engl.)