Respiratory chain

| Parent |
|
Electron transport chain Cell respiration Oxidative phosphorylation |
| Subordinate |
| Respiratory chain of the cell membrane Respiratory chain of the mitochondrial membrane |
| Gene Ontology |
|---|
| QuickGO |
| Parent |
|
Cell membrane mitochondrial membrane |
| Subordinate |
|
Complex I Complex II Complex III Complex IV |
| Gene Ontology |
|---|
| QuickGO |
The respiratory chain is part of the energy metabolism of most living things . On the one hand, the term respiratory chain denotes a metabolic pathway , namely a chain of biochemical redox reactions that take place one after the other , which the living beings use to generate energy, and on the other hand also the entirety of the protein complexes participating in the metabolic pathway .
The respiratory chain is a special case of an electron transport chain and, together with chemiosmosis, forms the process of oxidative phosphorylation .
Electrons supplied by NADH , FMNH 2 and FADH 2 are transferred to an oxidizing agent in a series of redox processes . So - especially with eukaryotes - the exergonic reaction of hydrogen (H 2 ) and oxygen (1/2 O 2 ) to water is divided into individual steps. Instead of a potentially explosive heat development, the released energy is used to synthesize the universal “energy currency” of the cell , ATP , from ADP and phosphate ( oxidative phosphorylation ). The hydrogen and the electron carriers NADH and FADH 2 bound electrons and the hydrogen bound thereto originate from the oxidation of external electron donors, for example - by means of the Krebs cycle - the degradation of fatty acids and glycolysis .
In eukaryotes the respiratory chain is located in the inner membrane of the mitochondria , in prokaryotes in the cell membrane . There are other electron donors than fats and sugars and other electron acceptors than oxygen.
Respiratory chain as an electron transport chain
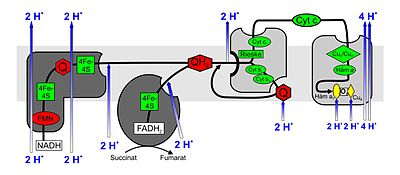
Electron transport chains consist of a number of redox molecules connected in series that are able to accept or release electrons. Via this chain electrons are passed from higher energy levels to lower ones, they fall downhill in steps, so to speak, with the individual redox molecules having an increasingly low energy level. In eukaryotes, the enzyme complexes I to IV and the hydrogen or electron carriers ubiquinone (coenzyme Q) and cytochrome c , which are embedded in the inner mitochondrial membrane, are involved in the reaction chain . The proteins involved in the electron transport chain (complexes I – IV) as well as the electron carriers ubiquinone and cytochrome c form a (complex) redox system .
Electron transport is associated with the uptake and release of protons. Through the spatial organization of these processes or by means of conformational changes in the protein structure caused by the flow of electrons , protons (H + ) are "transported" (real or as a net effect). This creates an "electrochemical proton gradient " (composed of the difference in concentration of the protons and the negative membrane potential inside the mitochondria generated by the removal of the positively charged protons). The energy of this “electrochemical proton gradient” ( proton motive force , “pmf”) is used by an ATP synthase through back diffusion of protons in the inner mitochondrial membrane , according to the now generally accepted chemiosmotic theory of Peter D. Mitchell , ATP from ADP and inorganic To synthesize phosphate (in rare cases fructose). This phosphorylation is called respiratory chain phosphorylation or oxidative phosphorylation (abbreviation: Oxphos ) because of the coupling to the respiratory chain .
Functions of the complexes of the respiratory chain
In addition to its outer membrane, a mitochondrion also contains an inner membrane . The space between these two membranes is called the intermembrane space (perimitochondrial space).
Three of the four complexes of the respiratory chain each span the inner mitochondrial membrane completely (integral), while complex II ends “blind” (peripheral). A proton concentration difference is generated between the intermembrane space and the interior ( matrix ) of the mitochondrion, which is then used in the ATP synthase to synthesize ATP.
Complex I.
NADH: ubiquinone oxidoreductase or NADH dehydrogenase . This huge enzyme complex (940 kDa ) reduced by NADH , particularly from citric , ubiquinone (UQ or Q) for Ubihydrochinon also ubiquinol (UQH 2 or QH 2 called). The complex consists of two parts, which together create its characteristic L-shape. Flavin-containing nucleotides ( FMN ) and iron-sulfur centers are required as prosthetic groups in one unit to catalyze the redox reaction. Due to the electron transport associated with the redox reactions, 3–4 protons per oxidized NADH are pumped into the intermembrane space. It is assumed that the coupling with the proton transport occurs through a conformational change of the enzyme.
Complex II
Succinate: ubiquinone oxidoreductase or succinate dehydrogenase . Complex II is the enzyme succinate dehydrogenase from the citric acid cycle. During the reaction in the citric acid cycle, succinate is oxidized to fumarate . FAD is a prosthetic group in the enzyme. It transfers its electrons in complex II to ubiquinone, which is reduced to ubihydroquinone. Complex II also contains iron-sulfur centers, such as complex I; however, no protons are pumped into the intermembrane space.
Complex III
Ubihydroquinone (ubiquinol): cytochrome c oxidoreductase or cytochrome c reductase . At complex III, the Q cycle contributes to the generation of the proton concentration difference through asymmetric absorption and release of protons. During the oxidation of ubiquinol (QH 2 ) to ubiquinone (Q), one molecule of cytochrome c is reduced in one cycle for each electron released (from ubiquinol) and two protons are released into the intermembrane space. The second electron reduces another ubiquinone to the free radical ubisemiquinone (QH) and then to QH 2 at another binding site on the mitochondrial matrix , whereby two protons are absorbed from the matrix.
After the two half-cycles, four protons per ubiquinol molecule are released into the intermembrane space, two protons are removed from the mitochondrial matrix and two cytochrome c are reduced. In complex III, a diversion from a two-electron transporter (ubiquinol) to a one-electron transporter (cytochrome c ) takes place.
Complex IV
Cytochrome c : O 2 oxidoreductase or cytochrome c oxidase . In complex IV, cytochrome c is oxidized and oxygen is reduced to water. The energy released is used to protons from the matrix space in the intermembrane space pump .
Cytochrome c is oxidized at complex IV and an electron is transferred to the complex. After the successive transfer of four electrons (e - ), a bound oxygen molecule can be reduced to two water molecules (H 2 O). The four protons (H + ) required for this are withdrawn from the matrix. The energy released during the reduction of oxygen to water is used by the enzyme to pump another four protons per oxygen molecule from the matrix via the inner mitochondrial membrane into the intermembrane space. This is done by changing the spatial structure: In a conformation, a protein has a high affinity for H + and therefore accepts a proton. In the opposite conformation, there is low affinity and the proton is released on the outside of the membrane.
Cytochrome c oxidase is a transmembrane protein with two heme a molecules (heme a and heme a 3 ) as prosthetic groups and two copper centers (Cu A and Cu B ) as cofactors . The enzyme is responsible for almost all oxygen consumption (formation of water from oxygen and hydrogen in the respiratory chain) of all oxygen-breathing organisms.
Respiratory chain inhibitors

A number of inhibitors have been identified that inhibit the electron transport chain at different points:
- Rotenone and amobarbital (Amytal) inhibit complex I (NADH oxidase). However, since the electron transfer from FADH2 to complex II is not influenced, the oxidative phosphorylation can still take place.
- Malonate and a number of fungicides (SDHI) inhibit complex II (succinate dehydrogenase).
- The antibiotic antimycin A inhibits complex III (cytochrome c reductase); it blocks the transfer of electrons from cytochrome b to cytochrome c1 ; the components of the respiratory chain in front of the site of action of antimycin A in complex III remain reduced, all behind remain oxidized. This inhibits the consumption of oxygen in complex IV and the synthesis of ATP in complex V. The fungicides from the class of strobilurins also inhibit complex III at point Q o .
- Cyanides , azides and carbon monoxide inhibit complex IV (cytochrome c oxidase); these molecules block the binding site for oxygen . As a result, this leads to an accumulation of electrons, as a result of which the components of the respiratory chain are completely reduced and the respiratory chain comes to a standstill.
- the antibiotic oligomycin inhibits ATP synthase by binding to its web (F o part), which means that the proton gradient is broken down much more slowly. As a result, the electron flow to maintain this gradient decreases significantly and the oxygen consumption decreases. Oligomycin also acts as a decoupler.
- Biguanides like the diabetes drug metformin presumably work by inhibiting complex I.
All of the previously mentioned inhibitors of the respiratory chain lead to a reduced consumption of oxygen. Inhibitors called decouplers behave differently.
Decoupler of the respiratory chain
Decouplers ( protonophores ) reduce the mitochondrial membrane potential (proton gradient). In doing so, they interrupt the link between oxidation and phosphorylation. As a result, the electron transport and the functioning of complexes I to IV take place completely, but the proton gradient built up during this process is canceled by the decoupler. This means that no synthesis of ATP can take place. Because protons no longer have to be translocated against an electrochemical gradient, the oxidation steps in complexes I to IV run much faster. At the same time, this leads to an increased consumption of oxygen.
- natural decouplers:
- Thermogenin , see also brown adipose tissue .
- artificial decouplers:
- 2,4-Dinitrophenol is a weak acid. It is protonated over the inner mitochondrial membrane due to the abundance of H + ions (lower pH ), can then pass through the membrane and is deprotonated intramitochondrially (higher pH).
- Carbonyl cyanide-m-chlorophenylhydrazone (CCCP)
- Carbonyl cyanide p -trifluoromethoxyphenylhydrazone (FCCP)
- Pentachlorophenol (PCP)
literature
- M. Saraste: Oxidative phosphorylation at the fin de siecle . In: Science , Vol. 283, 1999, No. 5407, pp. 1488-1493, PMID 10066163 .
- Löffler, Petrides: Human biochemistry . 7th edition.
Web links
- Oxidative phosphorylation - Reference pathway . KEGG (graphic representation of the mitochondrial respiratory chain complexes, English)
- Mitochondrial Pathways: Oxidative Phosphorylation . (English)
- Pedro Silva: The chemical logic behind ... Respiration and Fermentation . (English)
- Electron transport chain (animated illustration)
- Electron Transport Chain . Jassal / reactome
Individual evidence
- ↑ a b Bruce Alberts u. a .: Molecular biology of the cell . 4th edition. New York 2002, pp. 773-793
- ↑ RG Efremov, R. Baradaran, LA Sazanov: The architecture of respiratory complex I. In: Nature . Volume 465, number 7297, May 2010, pp. 441-445, doi: 10.1038 / nature09066 . PMID 20505720 .
- ↑ JM Berg, JL Tymoczko, L. Stryer: Biochemistry . 6th edition. Spektrum-Verlag, 2007.
- ↑ Bruce Alberts et al. a .: Molecular biology of the cell . 4th edition. New York 2002, p. 791
- ↑ Todd A. Swanson, Sandra I. Kim, Marc J. Glucksman: BRS Biochemistry, Molecular Biology, and Genetics . 5th edition. Lippincott Raven, 2010, ISBN 978-0-7817-9875-4 , p. 89.
- ↑ LS Huang, D. Cobessi et al. a .: Binding of the respiratory chain inhibitor antimycin to the mitochondrial bc1 complex: a new crystal structure reveals an altered intramolecular hydrogen-bonding pattern. In: Journal of molecular biology . Volume 351, number 3, August 2005, pp. 573-597, doi: 10.1016 / j.jmb 2005.05.053 . PMID 16024040 . PMC 1482829 (free full text).
- ↑ HR Bridges, VA Sirviö u. a .: Molecular features of biguanides required for targeting of mitochondrial respiratory complex I and activation of AMP-kinase. In: BMC biology. Volume 14, August 2016, p. 65, doi: 10.1186 / s12915-016-0287-9 , PMID 27506389 , PMC 4977651 (free full text).