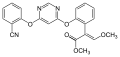
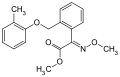
Strobilurins
Strobilurins are a class of natural products and their synthetic derivatives ( analogs ). Their name is derived from the mushrooms of the genus Strobilurus ( cone root ). Synthetically produced strobilurins have gained great importance as fungicidal active ingredients in crop protection .
history
The fungicidal effect of the ingredients of the pine cone carrot was discovered in the mid-1970s by Timm Anke ( Technical University of Kaiserslautern ). The elucidation of the chemical structure of the responsible strobilurin A was carried out by Wolfgang Steglich ( University of Bonn ). Since natural strobilurins decompose rapidly under the influence of light, the central double bond of their synthetic analogues has been replaced, for example by a phenyl ring . As a result, the photostability could be significantly improved. The first strobilurin fungicides were available in 1996. As early as 1999, strobilurins worth 600 million dollars were sold worldwide, which corresponded to a share of 10% of the fungicide market.
Natural substances
Strobilurins are naturally produced by fungi such as the pine cone and some other mushrooms (Basidiomycetes). The natural substances are generally referred to as strobilurin A , strobilurin B and so on, for strobilurin A there is the common name mucidin .

The crusty core mushroom ( Camarops lutea ) produces the strobilurins F, G and H. Strobilurin I was isolated from an Ethiopian mushroom species, strobilurin K from the winter helmling ( Mycena tintinnabulum ) and a New Zealand favolaschia species. The strobilurins D and G appear to be identical. The biosynthesis of strobilurins takes place via phenylalanine on the shikimic acid route. They have a fungicidal effect, some of them also have a cytostatic (strobilurin E) and antiviral effect .
Mode of action
Strobilurins work in the mitochondria of the fungus and inhibit cell respiration . This happens through the interruption of electron transport in the mitochondrial respiratory chain at the cytochrome bc 1 complex .
Toxicity and Ecotoxicity
The toxicity of strobilurins to plants and mammals is low. In warm-blooded animals , strobilurins are rapidly enzymatically converted to acid by cleavage at the ester group . Fish , water fleas and algae , on the other hand, are sensitive to strobilurins. Due to the rapid degradation of these substances in soil , sediments and water, no harmful effects are to be expected when used properly.
use
Strobilurins usually have a protective effect and must therefore be used preventively. To combat existing harmful fungi and to expand the spectrum of activity, they are often sold as combination preparations with fungicidal active ingredients from the azole class . The duration of action of the strobilurins is much longer than other fungicides at around four weeks.
Other effects
Treatment with strobilurins leads to a more intense green coloration of the leaves in plants, especially cereals . This is known as the "green effect". It is largely based on the fact that plants treated with strobilurins are less damaged by fungi or have to invest less energy in their defense. Further reasons could be an increased formation of growth-promoting phytohormones , a lowering of the CO 2 compensation point or a delay in aging processes. Strobilurins appear to delay the aging of plants by slowing down their protein and chlorophyll breakdown.
In the case of grain, on the one hand, this can lead to an increase in yield, since starch can be stored in the grain for longer . On the other hand, unripe, green straw can be threshed poorly , which is why strobilurins should not be used shortly before harvest . In practice, the additional yields as a result of the green effect are between 0 and 5 dt grain / ha and would not be worth using strobilurins on their own.
The use of strobilurins can have a disadvantageous effect on the chemothermal transformations (burning, smoldering) of the straw. With different ripening times of straw and grain, higher chlorine levels can be present in the straw at the time of harvest . This causes chlorine corrosion on the fittings of the incinerator and, in conjunction with alkali metals such as potassium and sodium, lowers the ash melting point, which leads to deposits during combustion or gasification.
Resistances
Numerous fungi have developed resistance to strobilurins so that they can no longer be effectively combated with them. A list of resistant fungi is published regularly by the Fungicide Resistance Action Committee .
Summary table of strobilurin fungicides
The following table contains the fungicidal active ingredients from the group of strobilurins currently available (as of February 2016). The columns D, A and CH show the approval in Germany, Austria or Switzerland.
| Surname | CAS number | Manufacturer | Application area | Trade names | Resistances | D. | A. | CH |
|---|---|---|---|---|---|---|---|---|
| Azoxystrobin | 131860-33-8 | Syngenta | broad spectrum | Amistar, Ortiva | X | X | X | |
| Dimoxystrobin | 149961-52-4 | BASF | Wheat and rapeseed cultivation | X | X | |||
| Fluoxastrobin | 361377-29-9 | Bayer | Grain cultivation | X | X | X | ||
| Kresoxim-methyl | 143390-89-0 | BASF | broad spectrum | Stroby, Discus | Apple scab (Lake Constance, Lower Elbe) | X | X | X |
| Metominostrobin | 133408-50-1 | Shionogi | Rice cultivation | |||||
| Orysastrobin | 248593-16-0 | BASF | Rice cultivation | |||||
| Picoxystrobin | 117428-22-5 | DuPont | Grain cultivation | Acanto | X | X | X | |
| Pyraclostrobin | 175013-18-0 | BASF | broad spectrum | X | X | X | ||
| Trifloxystrobin | 141517-21-7 | Bayer | broad spectrum | Twist / Gem / Swift, Flint | Apple scab (Lake Constance, Lower Elbe) | X | X | X |
Gallery of strobilurin structures
| Strobilurin | General structure | R 1 | R 2 | CAS number | PubChem |
|---|---|---|---|---|---|
| A. |

|
-H | -H | 52110-55-1 | 6437379 |
| B. | -OCH 3 | -Cl | 65105-52-4 | 6441102 | |
| C. | -OCH 2 -CH = C (CH 3 ) 2 | -H | 87081-57-0 | 6439808 | |
| F-1 | -OH | -H | |||
| F-2 | -H | -OCH 2 -CH = C (CH 3 ) 2 | |||
| H | -OCH 3 | -H | 129145-65-9 | 6441226 |
- Structures of commercial agents
Oudemansin structures gallery
| Oudemansin | General structure | R 1 | R 2 | CAS number | PubChem |
|---|---|---|---|---|---|
| A. |

|
-H | -H | 73341-71-6 | 6438712 |
| B. | -OCH 3 | -Cl | 87081-56-9 | 6440791 | |
| X | -H | -OCH 3 | 130640-32-3 | 6439301 |
Individual evidence
- ↑ Active ingredients and effect substances - From natural substances to pesticides ( Memento from March 15, 2008 in the Internet Archive ). BASF AG; Retrieved April 28, 2008.
- ↑ Bartlett et al .: Understanding the Strobilurin Fungicides . (PDF; 190 kB) In: Pesticide Outlook , August 2001, p. 143.
- ↑ Bernd Schäfer: Natural substances in the chemical industry , spectrum Akademischer Verlag, 2007, pp. 479-480, ISBN 978-3-8274-1614-8 .
- ↑ Veronika Hellwig, Johannes Dasenbrock, Dörte Klostermeyer, Stefan Kroiss, Tilman Sindlinger, Peter Spiteller, Bernd Steffan, Wolfgang Steglich, Michaela Engler-Lohr, Sylvia Semar, Timm Anke: New benzodioxepin type strobilurins from basidiomycetes. Structural revision and determination of the absolute configuration of strobilurin D and related beta-methoxyacrylate antibiotics . Tetrahedron , 1999, 55, 10101-10118, doi: 10.1016 / S0040-4020 (99) 00563-3
- ↑ a b c kresoxim-methyl . BASF brochure, around 1997.
- ↑ a b Wilhelm Bosse, Bernd Krieger: New fungicides in practice . In: DLG-Mitteilungen , 3/1998, pp. 50–54.
- ^ Jürgen Ceynowa, Henning Lindenberg: New means - new strategies . In: DLG-Mitteilungen , 2/1997, pp. 36–40.
- ↑ Dolores Fernández-Ortuño, Juan A. Torés, Antonio de Vicente, Alejandro Pérez-García: Mechanisms of resistance to QoI fungicides in phytopathogenic fungi (PDF; 149 kB). International Microbiology 11: 1-9, 2008.
- ^ FRAC: Publications , there under "List of Resistant Plant Pathogens".
- ^ Directorate-General for Health and Food Safety of the European Commission: EU pesticide database ; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on February 23, 2016.
- ↑ Anti-resistance strategies for scab control , accessed on April 20, 2012.