Azoles
Azoles , also known colloquially as pyrroles , are a group of heterocyclic chemical compounds . They are five - membered nitrogen - containing heteroaromatic compounds whose parent compound is pyrrole . The formal hydrogenation of one double bond gives pyrrolines , the formal hydrogenation of both double bonds leads to the pyrrolidines .
properties
Azoles are aromatic compounds. Five mesomeric boundary structures can be formulated. Azoles meet the Hückel criteria through the participation of the lone pair of electrons on the nitrogen atom. In terms of their aromaticity, they are between the more aromatic thiophenes and the practically no longer aromatic oxols .
Azoles have a basic character due to the lone pair of electrons on the nitrogen atom .
Electrophilic aromatic substitutions can be carried out on azoles . Because of the better stabilization of the intermediate stages of this reaction, these take place preferably in the 2-position. However , they are not stable to Lewis acids . These coordinate to the heteroatom and lead to ring opening.
Effects
Azoles are among the inhibitors of ergosterol synthesis . They work by inhibiting lanosterol micromasal demethalysis. This is followed by an inhibition of the conversion of lanosterol into ergosterol.
Manufacturing
Azoles can be prepared by Paal-Knorr synthesis from Di carbonyls such as di ketones or di aldehydes and ammonia .
Another possible synthesis is the Knorr pyrrole synthesis , for which aldehydes and α-aminoketones are required.
A one-pot reaction with substituted benzoins , 1,3-dicarbonyl compounds and ammonium acetate leads to tetrasubstituted pyrroles without a solvent or catalyst.
Azoles with Multiple Heteroatoms
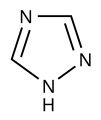
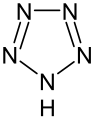
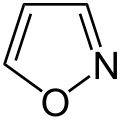
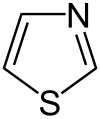
Pyrrole is not only the parent compound for the azoles, other heterocyclic groups of substances are also derived from this structure. These include the pyrazoles and imidazoles , which have two nitrogen atoms. The triazoles are known with three nitrogen atoms and the tetrazole with four . The azoles with heteroatoms of various types include the oxazoles and thiazoles .
the oxadiazoles
(1,2,4-oxadiazole)the thiadiazoles
(1,2,3-thiadiazole)
Occurrence and use
Like most heterocyclic compounds, pyrroles occur in numerous natural products . These include, for example, the porphyrins , which form the basis of heme . They also occur in other natural macrocyclic compounds such as corrin .
Azoles are contained as fragments in numerous active substances . In plastics, for example, azoles occur in the polypyrroles . More recent antimycotics from the group of inhibitors of ergosterol biosynthesis also belong to the azoles.
In 2013, azoles accounted for a third of all antifungal drugs sold for use in the agricultural sector. The massive use of azoles in agriculture is suspected to be responsible for the development of a resistant strain of Aspergillus and possibly also promoted the development of Candida auris , which was first detected in 2007 and dangerous to humans .
Individual evidence
- ^ A b D. T. Davies: Basistexte Chemie: Aromatic Heterocyclen , 1st edition, pp. 10-34, Wiley-VCH, Weinheim 1995, ISBN 3-527-29289-6 .
- ↑ Medical microbiology . 7th edition. Georg Thieme Verlag, Stuttgart 2019, ISBN 978-3-13-242355-8 , doi : 10.1055 / b-006-163249 ( thieme.de [accessed on August 26, 2020]).
- ↑ L. Knorr : Effect of the diacetuccinic acid ester on ammonia and primary amine bases , in: Ber. German Chem. Ges. 1885 , 18 , 299-311; doi : 10.1002 / cber.18850180154
- ↑ L. Knorr: Synthesis of pyrrole derivatives , in Ber. German Chem. Ges. 1884 , 17 , 1635-1642; doi : 10.1002 / cber.18840170220 .
- ^ Bhat, SI; Trivedi, DR: A catalyst- and solvent-free three-component reaction for the regioselective one-pot access to polyfunctionalized pyrroles in Tetrahedron Lett. 54 (2013) 5577-5582, doi : 10.1016 / j.tetlet.2013.07.153 .
- ^ E-EROS Encyclopedia of Reagents for Organic Synthesis , 1999–2013, John Wiley and Sons, Inc., entry for Ammonium acetate, accessed February 17, 2018 .
- ↑ Matt Richtel and Andrew Jacobs: "A Mysterious Infection, Spanning the Globe in a Climate of Secrecy" New York Times of April 6, 2018th