Paal-Knorr synthesis
The Paal-Knorr synthesis is a name reaction in organic chemistry that was first described in 1884 by Ludwig Knorr and Carl Paal . The process is used for the synthesis of five-membered heterocycles , i.e. substituted furans , thiophenes and pyrroles , starting from 1,4- diketones (γ-diketones).
Overview reactions
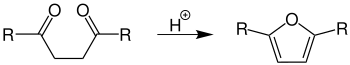
In the Paal-Knorr synthesis, 1,4-diketones are converted to heteroaromatic heterocyclopentadienes with either acid catalysts, amines or phosphorus (V) sulfide .
- The furan synthesis requires acid catalysts, 2,5-disubstituted furans are formed:
- For the preparation of 2,5-disubstituted thiophenes is phosphorus (V) sulphide requires:
In the compounds, the radicals R are various organyl radicals , e.g. B. alkyl radicals .
mechanism
Furan synthesis
In the first step, a carbonyl group of diketone 1 is protonated , whereby cation 2 is formed. In the next step, the oxygen atom of the other carbonyl group is nucleophilically added to the protonated carbonyl group with ring closure. At the same time, a proton splits off, creating the hydroxyfuran 3 :
The hydroxy group of the hydroxyfuran 3 is protonated, so that the reactive intermediate 4 is formed. This reacts further to furan 5 through dehydration and deprotonation :
Pyrrole synthesis
After Amaraths publication of the mechanism is probably as a direct introduction of a hemiaminal instead. This assumption is supported by his research results, according to which the stereochemical configuration of the starting compound is retained.
It can be assumed that initially there is a nucleophilic addition of the amine as a nucleophile to the carbon atom of a carbonyl group of diketone 1 . A reactive intermediate stage 2 is created , which reacts to ketoaminol 3 by proton transfer . This ketoaminol can also be referred to as hemiaminal if the nitrogen with the hydroxyl group is superficially considered.
In the next step, another nucleophilic addition takes place. The nitrogen of the aminol 3 is nucleophilically added to the second carbonyl group with ring closure and reacts to form a polar intermediate 4 . Diaminol 5 is formed by protonation of the oxygen atom with simultaneous deprotonation of nitrogen . The pyrrole derivative 6 is formed with dehydration .
Thiophene Synthesis
A possible mechanism for the synthesis of a thiophene was suggested by Foye.
First, the 1,4-diketone 1 is mixed with phosphorus (V) sulfide . Phosphorus pentasulfide is a thionation and dehydration reagent, which could promote the formation of furan. However, Foye has found experimentally that furan is not an intermediate in thiophene synthesis. When sulfur is introduced into the dicarbonyl compound 1 , it reacts to form thioketone 2 . The oxygen atom is protonated, so that the cation 3 is formed.
The sulfur attacks the carbon atom of the hydroxyl group in a nucleophilic manner , whereupon the heterocyclic thio-hemiacetal 4 is formed with deprotonation of compound 3 . Its elimination of water takes place with the formation of the 2,5-disubstituted thiophene 5 .
Individual evidence
- ^ Zero Wang: Comprehensive Organic Name Reactions an Reagents . 3 volumes. John Wiley & Sons, 2009, ISBN 978-0-471-70450-8 , pp. 2107 .
- ↑ Carl Paal : Synthesis of thiophene and pyrrole derivatives . In: Reports of the German Chemical Society . 18, No. 1, 1885, pp. 367-371. doi : 10.1002 / cber.18850180175 .
- ↑ Ludwig Knorr .: Action of the diacet succinic acid ester on ammonia and primary amine bases . In: Reports of the German Chemical Society . 18, No. 1, 1885, pp. 299-311. doi : 10.1002 / cber.18850180154 .
- ^ TL Gilchrist: Heterocyclic chemistry. ISBN 0-582-01421-2 .
- ^ László Kürti, Barbara Czakó: Strategic Applications of Named Reactions in Organic Synthesis . Elsevier Science & Technology Books, 2005, ISBN 0-12-369483-3 , pp. 498 .
- ^ Venkataraman Amarnath, Douglas C. Anthony, Kalyani Amarnath, William M. Valentine, Lawrence A. Wetterau, Doyle G. Graham: Intermediates in the Paal-Knorr synthesis of pyrroles . In: The Journal of Organic Chemistry . tape 56 , no. 24 , 1991, pp. 6924-6931 , doi : 10.1021 / jo00024a040 .
- ^ Adalbert Wollrab: Organic chemistry . Springer-Verlag, 1999, ISBN 3-540-43998-6 , pp. 850 .
- ^ A b E. Campaigne, William O. Foye: The Synthesis of 2,5-Diarylthiophenes . In: The Journal of Organic Chemistry . tape 17 , no. 10 , 1952, pp. 1405-1412 , doi : 10.1021 / jo50010a023 .