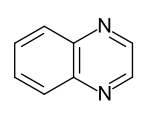
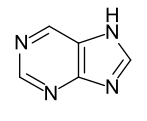
Heteroaromatics
The heteroaromatics are aromatic compounds whose ring structure contains one or more heteroatoms (e.g. nitrogen , oxygen , sulfur ). Formally, five- and six-ring heteroaromatics are derived from the cyclopentadienyl anion and from benzene by replacing a ring CH group ( methine group ) with a heteroatom.

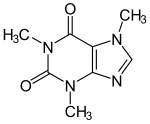

The heteroaromatics play an important role in natural product chemistry (examples: nicotine , caffeine ) and in pharmacy . So it concerns with antipyrine , the first synthetic antipyretic drugs (1887) by a five-membered heteroaromatic.
properties
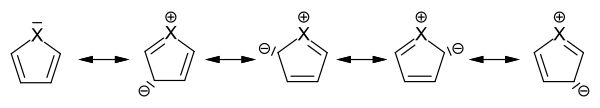
Monocyclic heteroaromatics are characterized by an electron sextet analogous to benzene (see Hückel rule ). They are divided into -electron-rich and -electron-poor heteroaromatics. In the case of the five-membered heteroaromatic compounds, the non-binding lone pair of electrons is part of the -electron sextet, which is distributed over the five ring atoms in the sense of the mesomeric boundary structures .
Mesomeric boundary structures in five-membered heteroaromatics
This increases the electron density on the ring carbon atoms at the expense of the hetero atom, so that the five-membered heteroaromatics belong to the electron-rich heteroaromatics.
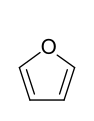
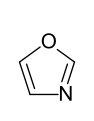
The electron-rich heteroaromatics are, as expected, more reactive than the aromatics without a heteroatom. However, the reactivity decreases with increasing group number and period of the hetero atom. So z. B. Pyrrole for auto-polymerization , while furan quickly forms brown oxides under the action of air , which can be thermally cleaved again. Thiophene, on the other hand, is already quite stable in the air. The typical reaction of the three compounds is electrophilic substitution . Only furan, the heterocycle with the lowest resonance energy, also shows typical diene reactions . Additional heteroatoms influence the stability depending on their position in the ring. For example, a nitrogen atom in the 3-position generally leads to a chemical stabilization of an electron-rich heteroaromatic (see cf. imidazole , oxazole , thiazole ).
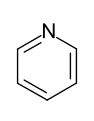
The six-ring heteroaromatics are -electron-poor heteroaromatics. The six-membered heteroaromatic pyridine only reacts under drastic conditions in the sense of an electrophilic substitution, but nucleophilic substitution reactions in the 2- and 4-position are quite easily possible.
Five-membered heterocycles with two heteroatoms such as the 1,2- diazoles (pyrazoles) and the 1,3-diazoles (imidazoles) are stronger bases than the weakly basic pyrrole because they contain an additional ring nitrogen atom whose lone pair of electrons is perpendicular to the system and that therefore hardly takes part in mesomerism.
Typical representatives
Monocyclic heteroaromatics
- Five-membered heteroaromatics with one heteroatom:
- Five-membered heteroaromatics with several heteroatoms:
- Six-ring heteroaromatics with one heteroatom:
- Six-ring heteroaromatics with several heteroatoms:
Bicyclic heteroaromatics
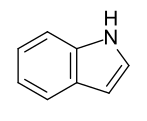
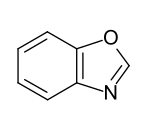
- Benzo-fused five-membered ring heteroaromatics:
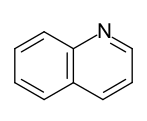
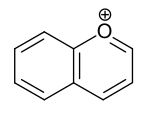
- Benzo-fused six-ring heteroaromatics:
- Heteroannelated heteroaromatics
See also
Individual evidence
- ↑ a b Olaf Kühl: Organic Chemistry . for biochemists, life scientists, physicians, pharmacists ... John Wiley & Sons, 2013, ISBN 978-3-527-66969-1 , pp. 52 ( limited preview in Google Book search).
- ^ A b Eberhard Breitmeier, Günther Jung: Organic chemistry . Basics, substance classes, reactions, concepts, molecular structure. 5th, revised edition. Georg Thieme Verlag, Stuttgart, New York 2005, ISBN 3-13-541505-8 , pp. 632 ff . ( limited preview in Google Book search).
- ↑ Jonathan Clayden, Nick Greeves, Stuart Warren: Organic Chemistry . 2nd ed. Oxford University Press, Oxford 2012, ISBN 978-0-19-927029-3 , pp. 723 ff . (English, limited preview in Google Book Search).
- ↑ a b Hans Peter Latscha, Uli Kazmaier, Helmut Alfons Klein: Organic Chemistry: Basic Chemistry II . Springer DE, 2008, ISBN 3-540-77107-7 , pp. 316 ff . ( limited preview in Google Book search).
- ^ Albert Gossauer: Structure and reactivity of biomolecules , Verlag Helvetica Chimica Acta, Zurich, 2006, p. 467, ISBN 978-3-906390-29-1 .