Phosphabenzene
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||
| General | |||||||||||||
| Surname | Phosphabenzene | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 5 H 5 P | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 96.07 g mol −1 | ||||||||||||
| Physical state |
liquid |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Phosphabenzene is an aromatic heterocycle that contains a phosphorus atom in addition to carbon and hydrogen . The molecular formula is C 5 H 5 P. It is the heavier homologue of pyridine . Other common names are phosphinine or phosphorine .
presentation
First Phosphabenzolderivat was in 1966 by Gottfried Märkl the 2,4,6-triphenylphosphabenzene described. The synthesis takes place by reacting 2,4,6-triphenylpyrylium tetrafluoroborate with tris (hydroxymethyl) phosphine in pyridine .
More Phosphabenzolderivate be prepared by reacting various Pyriliumjodide with tris (trimethylsilyl) phosphine accessible
A synthetic route to unsubstituted phosphabenzene was described by Arthur Ashe III in 1971. The coupling of propargyl bromide 1 with acetylene magnesium bromide 2 gives penta-1,4-diyne 3 , which is reacted with dibutyltin hydride to give 1,4-dihydro-1,1-dibutyltinbenzene 4 . The reaction of this intermediate with phosphorus tribromide gives the phosphine bromide 5 . The subsequent dehydrohalogenation with diazabicyclonones (DBN) gives the phosphabenzene 6 .
Francois Mathey, one of the world's leading phosphorus chemists, further developed Ashe's methods, including transition metal complex compounds including palladium and nickel-catalyzed coupling reactions.
properties
Physical Properties
Phosphabenzene has a planar structure that has about 88% of the aromaticity of benzene . The P – C bond length is 173 pm and the C – C bond lengths are in the range of 140 pm.
Chemical properties
Phosphabenzene is relatively stable, not very sensitive to air and moisture and can be stored for a long time under an inert gas atmosphere . In contrast, silabenzene , for example, is very sensitive to air and moisture and is thermally unstable. The stability of the title compound is related to the similar electronegativities of carbon (2.5) and phosphorus (2.1).
Although phosphabenzene is the heavy homologue of pyridine , there are crucial differences between these compounds. The lone pair of electrons of the N in pyridine is its HOMO , so that pyridine has good σ- donor properties.
The HOMO and LUMO of phosphabenzene, however, are its π and π * molecular orbitals , and the lone pair of electrons is located at a lower energy level. For this reason, phosphabenzene acts more as a σ acceptor and π donor ligand.
Pyridine reacts with nucleophiles predominantly in the C2 position because of the higher electronegativity of the nitrogen atom . In contrast, phosphabenzene forms λ 4 derivatives with nucleophiles , which in turn react with electrophiles to form λ 5 phosphabenzenes.
Homologues of phosphabenzene
The homologous compounds of heavier elements are known from pyridine and phosphabenzene. What they all have in common is their aromaticity. The stability decreases with the atomic number of the heteroatom . The bond lengths and angles of the heterobenzenes of the 15th group of the periodic table are shown below (from left to right: pyridine, phosphabenzene, arsabenzene , stibabenzene and bismabenzene ):
The instability, which increases with increasing atomic number, is related to the fact that a [4 + 2] cycloaddition preferably takes place at low temperatures and the isolation of the pure substances z. Sometimes designed impossible.
In electrophilic substitution reactions such as halogenation , acylation , etc., phosphabenzene behaves like other aromatic compounds.
Gottfried Märkl provided a compilation of properties, syntheses and reactions of the homologues phosphabenzene and arsabenzene in 1982.
Transition metal complexes
Many transition metal complexes are known of phosphabenzene. These also include mixed phosphazene / phosphabenzo ligands, which were investigated by Mathey. Phosphabenzene-rhodium systems appear to be superior to conventional catalysts in the hydroformylation of terminal and internal olefins.
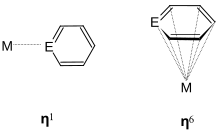
The Marburg organometallic chemist and book author Christoph Elschenbroich discusses in his book other phosphabenzene complex compounds in which the ligand functions as both η 1 and η 6 ligands. (Η 1 -C 5 H 5 P) -Mo (CO) 5 was already described by Ashe in 1977. Other transition metals tend to form complexes such as (η 6 -R n C 5 H 5-n P) n Cr (CO) 6-n , which was synthesized by Nöth in 1973.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ External identifiers of or database links for 2,4,6-triphenylpyrylium fluoroborate: CAS number: 448-61-3, EC number: 207-186-4, ECHA InfoCard: 100.006.535 , PubChem : 9930615 , ChemSpider : 8106246 , Wikidata : Q72514832 .
- ↑ External identifiers or database links for tris (hydroxymethyl) phosphine : CAS number: 2767-80-8, EC number: 220-445-6, ECHA InfoCard: 100.018.587 , PubChem : 76001 , ChemSpider : 68500 , Wikidata : Q27294320 .
- ↑ G. Märkl: 2,4,6-triphenylphosphabenzene . In: Angewandte Chemie . tape 78 , no. 18-19 , September 21, 1966, pp. 907 , doi : 10.1002 / anie.19660781817 .
- ↑ G. Märkl, F. Lieb, A. Merz: A new synthesis of derivatives of phosphabenzene . In: Angewandte Chemie . tape 79 , no. 10 , May 21, 1967, p. 475 , doi : 10.1002 / anie.19670791014 .
- ↑ Arthur J. Ashe, Paul Shu: 1-Phenylborabenzene anion . In: Journal of the American Chemical Society . tape 93 , no. 7 April 1971, p. 1804 , doi : 10.1021 / ja00736a052 .
- ↑ Arthur J. Ashe: Phosphabenzene and arsabenzene . In: Journal of the American Chemical Society . tape 93 , no. June 13 , 1971, p. 3293 , doi : 10.1021 / ja00742a038 .
- ↑ External identifiers of or database links to Penta-1,4-diyne : CAS number: 24442-69-1, PubChem : 141112 , ChemSpider : 124473 , Wikidata : Q83049706 .
- ^ Trinity University Cheminformatics: Distances About the Ring ( Memento of March 10, 2005 in the Internet Archive ).
- ↑ Ashe III, Smith: The reaction of phosphabenzene, arsabenzene and stibabenzene with methyllithium .
- ↑ Christoph Elschenbroich: Organometallchemie . 6th edition. Teubner Verlag, Wiesbaden 2008 ( limited preview in the Google book search [accessed on February 25, 2010]).
- ↑ Gottfried Märkl: Phosphabenzol and Arsabenzol. The higher element homologues of pyridine , Chemie in our time 16 (1982) 139-148, doi : 10.1002 / ciuz.19820160503 .
- ↑ Sava, Mézailles, Maigrot, Nief, Ricard, Mathey, Floch: A Versatile Approach toward Phosphinine-Phosphole-Based and Phosphinine-Phosphaferrocene-Based Tridentate Ligands .
- ↑ Lothar Weber: Phosphorus heterocycles: from laboratory curiosities to ligands in highly efficient catalysts. In: Angewandte Chemie. 114, 2002, pp. 583-592, doi : 10.1002 / 1521-3757 (20020215) 114: 4 <583 :: AID-ANGE583> 3.0.CO; 2-2 .
literature
- Christoph Elschenbroich , A. Salzer: Organometallchemie . Teubner Taschenbücher Chemie 1990 , ISBN 3-519-23501-3
- Louis D. Quin: A Guide to Organophosphorus Chemistry . Wiley-Interscience 2000 , ISBN 0-471-31824-8