Heterocycles
Heterocycles (from Greek ἕτερος heteros 'different', 'foreign' and Latin cyclus 'circle'; but also: heterocycles ) are cyclic chemical compounds with ring-forming atoms made up of at least two different chemical elements . The term is mainly used in organic chemistry and describes a ring-shaped organic compound whose ring structure contains at least one atom of another chemical element in addition to carbon atoms . These foreign atoms are called heteroatoms . A ring structure can contain one or more identical or different heteroatoms. The most common are nitrogen , oxygen and sulfur . Aromatic heterocycles are also referred to as heteroaromatics for short .
In organic chemistry, the heterocycles form an extensive class of compounds. They can be classified according to the type and number of heteroatoms as well as the ring size and degree of saturation of the cyclic system. By considering the degree of saturation, the classification into saturated heterocycloalkanes (compare cycloalkanes ), partially unsaturated heterocycloalkenes (compare cycloalkenes ) and the heteroaromatics results . Cycloalkynes (compare alkynes ) are seldom found, especially in macrocycles .
history
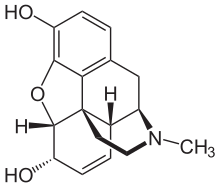
The first heterocyclic compounds obtained as pure substances were natural substances that could be isolated from plant material in the 19th century . Due to the lack of analytical methods at that time, research progress often took decades. So 1806 was first morphine from Sertürner in crystalline receive the form, to determine the correct molecular formula but the compound passed 42 years and only 77 years after its discovery was the structure of the compound to be finally resolved. A number of nitrogenous heterocyclic bases could be obtained by Anderson from animal material. The isolated substances included pyridine and picolines , the structures of which could be elucidated decades later. Körner postulated the hypothesis that there was an analogy between benzene and naphthalene as well as pyridine and quinoline , in the structures of the former only one CH unit had to be replaced by a nitrogen atom.
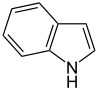
The first synthesis of a heterocyclic compound was achieved by William Ramsay in 1877. He observed the formation of pyridine when acetylene and hydrogen cyanide gas were passed through a red - hot pipe. In the same year, Baeyer and Caro were able to obtain indole by reacting N -ethylaniline in a red-hot pipe. Just two years later, Königs achieved the first synthetic preparation of quinoline by converting N -allylaniline onto red-hot lead oxide .
In the course of the advancing understanding of the structure of heterocyclic compounds, rules for their nomenclature were drawn up independently by Arthur Hantzsch and Oskar Widman in 1887 and 1888. These have been expanded over the years, but still form the basic structure of today's Hantzsch-Widman nomenclature .
Nomenclature, stem systems and connections
The IUPAC recommended the use of the Hantzsch-Widman system for the nomenclature of heterocyclic systems . However, other nomenclatures are also used; Colloquially, trivial names are often used. For a detailed discussion of the official nomenclature, please refer to the main article on the Hantzsch-Widman system .
Heterocycles with elements of the fifth and sixth main group
| Simple heterocycles with one heteroatom | |||||||
|---|---|---|---|---|---|---|---|
| Saturated heterocycles | Unsaturated heterocycles | ||||||
| Heteroatom | nitrogen | oxygen | sulfur | nitrogen | oxygen | sulfur | |
| 3 rings | |||||||
| Nomenclature name | Aziridine | Oxirane | Thiiran | Azirine | Oxiren | Thiren | |
| Common name | Ethyleneimine | Ethylene oxide | Ethylene sulfide | - | Acetylene oxide | Acetylene sulfide | |
| structure | 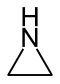 |
 |
 |
 |
 |

|
|
| 4 rings | |||||||
| Nomenclature name | Azetidine | Oxetane | Thietan | Acet | Oxetium ion | Thietium ion | |
| Common name | 1,3-propyleneimine | Trimethylene oxide | Trimethylene sulfide | Azacyclobutadiene | - | - | |
| structure |  |
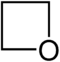 |
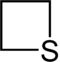 |
 |
 |

|
|
| 5 rings | |||||||
| Nomenclature name | Azolidine | Oxolane | Thiolane | Azole | Oxol | Thiol | |
| Common name | Pyrrolidine | Tetrahydrofuran | Tetrahydrothiophene | Pyrrole | Furan | Thiophene | |
| structure | 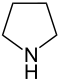 |
 |
 |
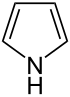 |
 |
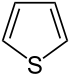
|
|
| 6 rings | |||||||
| Nomenclature name | Azinane | Oxane | Thian | Azine | Oxinium ion | Thiinium ion | |
| Common name | Piperidine | Tetrahydropyran | Tetrahydrothiopyran | Pyridine | Pyrylium ion | Thiopyrylium ion | |
| structure |  |
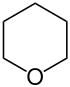 |
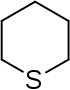 |
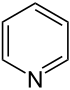 |
 |

|
|
| 7 rings | |||||||
| Nomenclature name | Azepan | Oxepan | Thiepan | Azepine | Oxepin | Thiepin | |
| Common name | Hexamethyleneimine | Hexamethylene oxide | Hexamethylene sulfide | Azatropilids | Oxacycloheptatriene | Thiotropilids | |
| structure |  |
 |
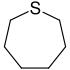 |
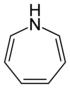 |
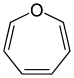 |

|
|
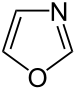
A number of other heterocycles are also of great importance and are found widely. These include both compounds that have several heteroatoms and those that consist of polynuclear ring systems. The most prominent parent systems are five-membered nitrogen-containing unsaturated heterocycles such as pyrazole , imidazole and indole as well as the six-membered rings quinoline , isoquinoline , purine , pyrimidine and saturated piperidine . Furthermore, oxazoles , thiazoles and thiazines are also frequently encountered.
While the majority of the heterocyclic compounds have oxygen, nitrogen or sulfur as heteroatoms, other elements are rarely found. Thus phosphabenzene.l'l and arsabenzene as homologues known of pyridine. Also phosphole and Arsol as Pyrrolhomologe could be synthesized.
Metallacycles
Not only main group elements can function as heteroatoms, subgroup elements can also be built into cyclic systems. Examples are the cyclopentene derivative in Hoveyda catalysts , which contains an oxygen atom and a ruthenium atom. Di cobalt derivatives of cyclobutane are formed in the Pauson-Khand reaction and in the Nicholas reaction .
A specialty are the Metallabenzole and Metallabenzine . These are unsaturated six-membered heterocycles that have fragments of transition metal complexes in their ring structure. The existence of such systems was predicted by Thorn and Hoffmann in 1979 . The first compound in this class was an Osma benzene three years later . It could be shown that this is an aromatic system that enters into substitution reactions similar to benzene. Analogous to gasoline , it was also possible to represent metal awnings that have a triple bond in a six-membered ring. In contrast to gasoline, however, these do not enter into any addition reactions at the triple bond.
Structure of the Hoveyda Catalyst I; Cy = cyclohexyl
- → For further heterocyclic compounds see also category: Heterocyclic compound .
Occurrence
Heterocyclic compounds are a widespread class of compounds in nature. They have important functions in biological processes, which they usually take on because of their ability to form complexes, and more rarely because of their Brønsted basicity. In the case of alkaloids , the most prominent representatives of which are atropine , cocaine , caffeine and nicotine, as well as opiates , they occur as natural toxins that can also be misused as intoxicants . Some members of this group are used in medicine . Three of the natural amino acids , namely proline (pyrrolidine derivative), histidine (imidazole derivative) and tryptophan (indole derivative), also have heterocyclic rings.
DNA
In DNA , the role of nucleobases is taken over by heterocyclic compounds. The nucleobases of DNA and RNA have a purine ( adenine , guanine , xanthine and hypoxanthine ) or pyrimidine basic body ( cytosine , thymine and uracil ). Their basic properties allow the formation of hydrogen bonds between the complementary base pairs and thus form the bonds between the two single strands of DNA. A medical application arises from the ability of the nucleic bases to form complexes. For example, the effect of the cytostatic agent cisplatin , which is used for cancer therapy , is based on the complexation of the platinum complex used with the N 7 atom of guanine and adenine. The resulting cross-links between the individual strands impede cell metabolism and usually lead to cell death, which prevents the reproduction of cancer cells. The coenzymes NAD , NADP and ATP also have these basic bodies.
Cytochromes
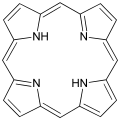
Under cytochromes the group of color is proteins summarizes which malice as prosthetic groups possess. These occur in some biologically important processes. This includes the transport of oxygen, which is brought about in the blood by the red blood pigment hemoglobin and in the muscle tissue by the myoglobin . In photosynthesis and cellular respiration , they occur as well in appearance as in many enzymes , such as in the cytochrome P450 superfamily. The heme group is made up of derivatives of porphyrin , which complexes various metal ions . Porphyrins are macrocyclic compounds that are formally composed of four pyrrole units. The macrocycle appears here as a tetradentate chelate ligand and, due to the macrocyclic effect , is able to form thermodynamically stable but kinetically labile complexes with ions that are difficult to complex, such as magnesium . This variable system ensures that a large number of ions can be used depending on the requirements. In the case of hemoglobin and myoglobin, these are iron ions, while a magnesium cation is bound in chlorophyll, which does not belong to the cytochromes .
Cobalamins
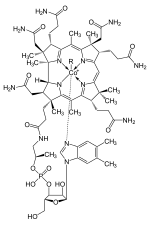
The group of cobalamins , important coenzymes with their most prominent representative vitamin B 12 , are cobalt complexes, the metal ion of which is complexed by a macrocyclic corrin ring . Similar to the porphyrin ring, the corrin ring is formally composed of four pyrrole units, but is shorter by one bridge member. This leads to a narrower ring opening, which allows the complexation of smaller metal ions. One axial coordination site is occupied by a histidine ligand. The heteromacrocycle again takes on the role of a complexing agent that forms both thermodynamically stable and kinetically inert complexes. In this case, however, it remains flexible enough that it is able to take up ions of an entire size range, in this case both mono- and di- and trivalent cobalt. The flexibility is determined by the twist of the planar ligand in the plane or through the arching ( doming reached).
Natural colors and fragrances
Indigo is a vegetable dye that was used to dye fabrics over 4000 years ago. It is obtained from plants such as Indigofera arrecta , Indigofera tinctoria or Indigofera suffruticosa . However, these do not contain indigo, but indican , a glucoside of indoxyl , from which indigo is chemically obtained. This is done by hydrolysis of the glucoside, whereby the yellow indoxyl is initially formed, which is then converted oxidatively to indigo. Both indigo and indican have an indole basic structure. Indican itself is colorless, while indigo itself has a characteristic blue color.
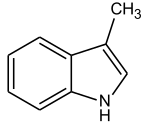
In Skatol is another indole derivative, namely 3-methyl indole. Skatole is one of the main odorous substances in human and animal feces. Also civet , which is used for perfumery, contains small amounts of skatole. The substance has a very low odor threshold and can therefore still be perceived in a very high dilution. So 0.23 µg per kg of starch are still perceptible to humans. The biosynthesis takes place via a two-stage oxidation of tryptophan with subsequent decarboxylation . Since animal tissue has a higher tryptophan concentration, the feces of carnivorous animals usually have a stronger smell than that of herbivorous species.
properties
No general statements can be made about the chemical and physical properties of heterocycles, but they can be classified and treated in groups according to the type of hetero atom and the degree of saturation. It makes sense to distinguish between aromatic and unsaturated heterocycles.
Aromaticity
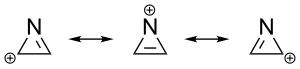
The aromaticity reflects the property of aromatic compounds to form a ring-shaped system of delocalized π-electrons . Just like benzene as a classic example of an aromatic, most unsaturated heterocycles that meet the Hückel criteria have aromatic properties. The azirine cation is the simplest aromatic heterocycle. It only has one pair of π electrons and its aromatic character is due to the delocalization of the positive charge. In contrast, the corresponding anion is a classic anti-aromatic .
In Hückel heteroaromatics, the aromaticity is strongly dependent on the electronegativity of the heteroatoms. If these have a high electronegativity, a higher part of the electron density is localized at these nuclei, which reduces the aromaticity of the system. This can be observed, for example, in the series of five-membered heterocycles. While the nitrogen-containing pyrrole shows good aromatic properties, these decrease sharply with increasing electronegativity of the heteroatom via thiophene to furan. The aromaticity of furan is so weak that it even enters into Diels-Alder reactions , a behavior that is atypical for aromatics, since the reaction is accompanied by the loss of mesomerism stabilization .
Basicity
Frequently encountered heteroatoms usually have at least one free electron pair. This gives them both a Brønsted and a Lewis basic character. For example, pyridine has a pK b value of 8.94 at 20 ° C. If there are several electron-withdrawing heteroatoms, the basicity is reduced , since they compete for electron density and can therefore only combine a smaller amount on themselves. Accordingly, six-membered heteroaromatics which carry two nitrogen atoms in the ring have higher pK b values than pyridine ( pyridazine 11.71, pyrimidine 12.87, pyrazine 13.6 at 20 ° C. ).
Due to their Lewis basicity, heterocyclic compounds can also function as ligands in complexes . Saturated heterocycles are usually classic σ-donor ligands, while heteroaromatic compounds are also π-acceptor ligands . Due to the ability to form back bonds from the metal center into the LUMO of the ligand, they usually cause a high level of ligand field splitting and form stable complexes.
synthesis
Nitrogen-containing heterocycles
Azirines can be obtained photochemically from vinyl azides in good yield. The saturated aziridines are accessible via an intramolecular substitution. For this purpose, amines which have a leaving group in the β position, for example 2-bromoethylamine , are used in a basic medium.
Pyrrole derivatives can be obtained in a simple way by the Paal-Knorr synthesis . Here, 1,4-dicarbonyl compounds are reacted with ammonia or a primary amine . The saturated and partially unsaturated pyrrolidines can usually be obtained from these by hydrogenation.
The Hantzsch pyridine synthesis exists as a simple synthetic access route for the frequently encountered group of pyridines . This is a sequence of condensation reactions and isomerizations through which a dihydropyridine species is initially formed from ketones , aldehydes and ammonia, which can then be oxidized to the pyridine derivative using nitric acid .
Oxygenated heterocycles
The Prileschajew reaction is usually used for the synthesis of epoxides . This is based on alkenes which are oxidized by means of peroxycarboxylic acids . For this purpose, peracids substituted with electron-withdrawing groups are used, mostly meta- chloroperbenzoic acid . The enantioselective synthesis of epoxides, the using Sharpless epoxidation be carried out for their development, among others, the 2001 Nobel Prize in Chemistry to Barry Sharpless was awarded.
Oxetanes can be obtained synthetically by the Paternò-Büchi reaction . For this [2 + 2] cycloaddition , an alkene is made to react photochemically with a carbonyl compound .
The furan produced annually on a multi-ton scale can be obtained on an industrial scale from the reaction between acetylene and formaldehyde and subsequent conversion of the diol thus obtained with ammonia. The saturated tetrahydrofuran (THF) and partially unsaturated derivatives can be prepared from the furan derivatives formed by hydrogenation .
Sulfur-containing heterocycles
Thirenes, the simplest unsaturated sulfur-containing three-membered heterocycles, are unstable and, for this reason, are found only very rarely. For example, they can be obtained from thioacetaldehyde by a photochemical reaction . For the saturated thiiranes, however, a large number of synthetic routes are described in the literature. A simple access is given by a substitution reaction of sulfides on α, β-dibromoalkanes.
3-Thiazolines can be synthesized in a multi-component reaction - the Asinger reaction or reactions derived from it.
Thiophenes and their saturated derivatives are the most common sulfur-containing heterocycles. Like its nitrogen and oxygen analogues, thiophene can be obtained from a 1,4-dicarbonyl compound by a Paal-Knorr synthesis. The sulfur atom in this case is introduced through the use of hydrogen sulfide or phosphorus pentasulfide . Substituted thiophenes can be synthesized using the Gewald reaction .
Reactions
Aromatic substitution
Since a π complex is initially formed between electrophile and aromatic in electrophilic aromatic substitutions , the success of the reaction depends on the π electron density of the aromatic. In heteroaromatics, in which the lone pair of electrons of the heteroatom is not involved in the π system, the π electron density is usually lower compared to the pure carbon aromatic. The formation of the π-complex is therefore less preferred and an electrophilic substitution is made more difficult (electron-poor heteroaromatic compounds, example: pyridine ). On the other hand, the electron density is increased when the lone pair of electrons of the heteroatom is involved in the π-system and thus facilitates the electrophilic substitution ( electron excess heteroaromatic , example: pyrrole ).
With regard to the regioselectivity , heteroatoms of electron-poor heteroaromatic compounds can be treated in a similar way to substituents with electron-withdrawing character. With regard to the six-membered heteroaromatics, this means that an electrophilic substitution will preferably take place in the β-position to the heteroatom. The reason for this is less to be sought in the electron density at this position, but is largely due to the fact that there is no mesomeric boundary structure in the intermediate σ complex that has a positive charge on the electronegative heteroatom. Analogously, it can be justified that electron-rich heteroaromatics are preferably substituted in the α-position to the heteroatom.
Weak electrophiles usually react poorly with electron-poor heteroaromatics. Systems with greatly reduced electron density, for example those with several heteroatoms or electron-withdrawing substituents, often do not react at all in the sense of an electrophilic substitution, but only in a nucleophilic aromatic substitution. An example of this is the Tschitschibabin reaction for the synthesis of α-aminopyridines.
Ring opening

Some, mostly saturated, heterocycles can be opened by reaction with suitable nucleophiles. This ring opening is facilitated by the presence of Lewis acids. An example of this is the opening of thietane by bromine .
Epoxides can also be opened by a nucleophile. If the resulting alcoholate is not sufficiently stabilized, it functions as a nucleophile for further ring opening. This is followed by polymerization to form a polyether .
The opening of epoxies is used in a number of chemical reactions. Thus, the epoxide at the will dihydroxylation for the production of trans - diols with sodium hydroxide or potassium hydroxide opened, an intramolecular electron-hole by displacement, in the Eschenmoser fragmentation utilized.
use
Heterocyclic compounds can be found practically everywhere, so that a detailed study would fill several volumes. The majority of active pharmaceutical ingredients are made up of heterocycles. The best-selling preparations include atorvastatin (Sortis ® , Lipitor ® ), sildenafil (Viagra ® ) and diazepam (Valium ® ). The total annual sales volume of these drugs is almost 100 billion euros.
In preparative chemistry
As bases
In preparative chemistry, aromatic or partially saturated nitrogen-containing heterocycles are predominantly used as bases. They are often used for the deprotonation of alcohols or strongly C, H-acidic compounds. In addition to pyridine, this is the main area of application for the bases DBU and DBN . Their advantage lies in the base strength, which is weaker compared to organometallic bases such as lithium alkyls or hydrides . This ensures a higher selectivity. Furthermore, they are usually only weak nucleophiles, so they usually do not attack carbonyl compounds, which increases the choice of possible substrates.
As a solvent
Heterocyclic compounds can also be found among the solvents frequently used . This includes above all tetrahydrofuran (THF), which is used as a weakly polar and weakly coordinating ether . Pyridine and very rarely also pyrimidine and piperidine are used as basic solvents. Large-scale use of the sulfur cycle sulfolane was developed by the mineral oil company Shell in the 1960s. This is used to purify raw products from aromatic compounds in a liquid-liquid- extractive process.
In electrical engineering

Heterocyclic compounds can have current-conducting properties and can therefore be used as a replacement for conventional metallic conductors in electronic components. They can be used as electrical conductors , semiconductors or superconductors, for example in batteries , transistors and light emitting diodes . Compounds that have an extensive conjugated π-electron system, such as polythiophenes or polypyrroles, are suitable for this purpose . Single crystals of tetrathiafulvalene (TTF) and tetracyanoquinodimethane (TCNQ) have also shown interesting properties .
Individual evidence
- ↑ Entry on heterocyclic compounds . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.H02798 Version: 2.1.5.
- ↑ a b c d e f g h H. Beyer, W. Walter: Textbook of organic chemistry. 23rd edition. S. Hirzel Verlag, Stuttgart 1998, ISBN 3-7776-0808-4 , pp. 753-876.
- ↑ Th. V. Anderson: About picoline. A new base made from coal tar oil. In: Liebigs Ann. 60, 1846, pp. 86-103; doi: 10.1002 / jlac.18460600106 .
- ↑ Th. V. Anderson: Products of the dry distillation of animal matter. In: Liebigs Ann. 70, 1849, pp. 32-38; doi: 10.1002 / jlac.18490700105 .
- ↑ Th. Anderson: About the products of the dry distillation of animal matter. In: Liebigs Ann. 80, 1851, pp. 44-65; doi: 10.1002 / jlac.18510800104 .
- ↑ Th. Anderson: About the products of the dry distillation of animal matter. In: Liebigs Ann. 94, 1855, pp. 358-365; doi: 10.1002 / jlac.18550940312 .
- ^ A. Ladenburg : Lectures on the history of the development of chemistry since the time of Lavoisier. English translation of a lecture. Full text access (PDF; 5.0 MB).
- ↑ About W. Ramsay's discovery in: Ber. German Chem. Ges. 10, 1877, p. 736; doi: 10.1002 / cber.187701001202 .
- ↑ A. Baeyer, H. Caro: Indol aur Äthylanilin. Ber. German Chem. Ges. 10, 1877, pp. 692-693; doi: 10.1002 / cber.187701001193 .
- ↑ W. Königs: Synthesis of quinoline from allylaniline. In: Ber. German Chem. Ges. 12, 1879, p. 453; doi: 10.1002 / cber.187901201128 .
- ↑ a b A. Hantzsch , JH Weber: About compounds of thiazole (pyridine of the thiophene series). In: Ber. German Chem. Ges. 20, 1887, pp. 3118-3132; doi: 10.1002 / cber.188702002200 .
- ↑ a b O. Widman: On the nomenclature of compounds which contain nitrogen nuclei. In: J. Prakt. Chem. 38, 1888, pp. 185-201; doi: 10.1002 / prac.18880380114 .
- ^ A b W. H. Powell: Revision of the extended Hantzsch-Widman System of Nomenclature for Heteromonocycles. In: Pure Appl. Chem. 55, 1983, pp. 409-416; doi: 10.1351 / pac198855020409 .
- ↑ G. Märkl: Phosphabenzol and Arsabenzol. In: Chemie in our Zeit 16, 1982, pp. 139-148; doi: 10.1002 / ciuz.19820160503 .
- ↑ Christoph Elschenbroich : Organometallchemie. 5th edition. Teubner, Wiesbaden 2005, ISBN 3-519-53501-7 , pp. 220-221.
- ↑ T. Laue, A. Plagens: Name and keyword reactions. 4th edition. Teubner, Wiesbaden 2004, ISBN 3-519-33526-3 .
- ^ A b G. P. Elliott, WR Roper, JM Waters: Metallacyclohexatrienes or Metallabenzenes. Synthesis of Osmabenzene Derivates and X-ray Structure of [Os (C (S) CHCHCHCH) (CO) (PPh 3 ) 2 ]. In: J. Chem. Soc., Chem. Comm. 1982, pp. 811-813; doi: 10.1039 / C39820000811 .
- ↑ a b Christoph Elschenbroich : Organometallchemie. 5th edition. Teubner, Wiesbaden 2005, ISBN 3-519-53501-7 .
- ↑ G. Jia: Progress in the Chemistry of Metallabenzynes. In: Acc. Chem. Res. 37, 2004, pp. 479-486; doi: 10.1021 / ar0201512 .
- ↑ E. Breitmaier: Alkaloids: narcotics, hallucinogens and other active ingredients, lead structures from nature. 2nd Edition. Teubner Verlag, Wiesbaden 2002, ISBN 3-519-13542-6 .
- ^ G. Löffler, PE Petrides: Physiologische Chemie. 4th edition. Springer, Berlin 1988, ISBN 3-540-13199-X , pp. 69-72.
- ↑ W. Voigt, A. Dietrich, H.-J. Schmoll: Cisplatin and its analogues. In: Pharmazie in our time 35, 2006, pp. 134-143; doi: 10.1002 / pauz.200500162 .
- ↑ M. Galanski, BK Keppler: Tumor-inhibiting metal compounds. In: Pharmazie in our time 35, 2006, pp. 118-122; doi: 10.1002 / pauz.200500160 .
- ^ Lehninger: Biochemistry. 3. Edition. Springer, Berlin 2001, ISBN 3-540-41813-X , pp. 716-726.
- ^ G. Löffler, PE Petrides: Physiologische Chemie. 4th edition. Springer, Berlin 1988, ISBN 3-540-13199-X .
- ^ W. Kaim , B. Schwederski: Bioinorganische Chemie. 4th edition. Teubner, Wiesbaden 2005, ISBN 3-519-33505-0 , pp. 26-29.
- ↑ a b W. Kaim , B. Schwederski: Bioinorganische Chemie. 4th edition. Teubner, Wiesbaden 2005, ISBN 3-519-33505-0 .
- ↑ GN Schrauzer: Recent developments in the field of vitamin B 12 : reactions of the cobalt atom in corrin derivatives and vitamin B 12 model compounds. In: Angew. Chem. 88, 1976, pp. 465-474; doi: 10.1002 / anie.19760881403 .
- ↑ HR Christen, F. Vögtle: Organic chemistry - From the basics to research. 2nd Edition. Otto Salle Verlag, Frankfurt am Main 1996, ISBN 3-7935-5398-1 , pp. 14-15.
- ↑ W. welding Heimer: perfumes of animals. In: Fette, Seifen, Anstrichmittel 67, 1986, pp. 381-382.
- ↑ a b H.-D. Belitz , W. Grosch, P. Schieberle: Lebensmittelchemie , 2001, 5th edition. Springer-Verlag, ISBN 978-3-540-41096-6 , p. 381.
- ↑ a b c d e f D. T. Davies: Basic Texts Chemistry: Aromatic Heterocyclen. 1st edition. Wiley-VCH, Weinheim 1995, ISBN 3-527-29289-6 .
- ↑ a b D'Ans-Lax: Pocket book for chemists and physicists. 3. Edition. Volume 1, Springer-Verlag, Berlin-Göttingen-Heidelberg 1967 ( ChemieOnline - pK b and pK s values ).
- ^ L. Gade: Coordination Chemistry. 1st edition. VCH, Weinheim 1998, ISBN 3-527-29503-8 .
- ↑ K. Banert: Reactions of Unsaturated Azides, 8. Azidobutatrien and Azidobutenines. In: Chem. Ber. 122, 1989, pp. 1175-1178; doi: 10.1002 / cber.19891220624 .
- ↑ C. Paal: Synthesis of thiophene and pyrrole derivatives. In: Ber. German Chem. Ges. 18, 1885, pp. 367-371; doi: 10.1002 / cber.18850180175 .
- ↑ A. Hantzsch : Condensation products from aldehyde ammonia and ketone-like compounds. In: Ber. German Chem. Ges. 14, 1881, pp. 1637-1638; doi: 10.1002 / cber.18810140214 .
- ↑ N. Prileschajew: Oxidation of unsaturated compounds using organic superoxides. In: Ber. German Chem. Ges. 42, 1909, pp. 4811-4815; doi: 10.1002 / cber.190904204100 .
- ↑ Reinhard Brückner: reaction mechanisms. 3. Edition. Spektrum Akademischer Verlag, Munich 2004, ISBN 3-8274-1579-9 , pp. 138-144.
- ↑ G. Büchi, CG Inman, ES Lipinsky: Light-catalyzed Organic Reactions. I. The Reaction of Carbonyl Compounds with 2-Methyl-2-Butene in the Presence of Ultraviolet Light. In: J. Am. Chem. Soc. 76, 1954, pp. 4327-4331; doi: 10.1021 / ja01646a024 .
- ↑ T. Laue, A. Plagens: Name and keyword reactions. 4th edition. Teubner, Wiesbaden 2004, ISBN 3-519-33526-3 , pp. 261-263.
- ↑ Karsten Krohn, Ulrich Wolf: Brief introduction to the chemistry of heterocycles . Teubner Study Books Chemistry, 1994, ISBN 978-3-519-03532-9 ( Springer-Link ).
- ↑ G. Maier, U. Flögel, HP Reisenauer, B. Hess, LJ Schaad: Cleavage of HCl from ethanesulfenyl chloride and chlorodimethyl sulfide. In: Chem. Ber. 124, 1991, pp. 2609-2612; doi: 10.1002 / cber.19911241134 .
- ↑ J. Delepine in: CR Hebd. Seances Acad. Sci. 172, 1921, p. 158.
- ↑ Friedrich Asinger and Manfred Thiel: Simple syntheses and chemical behavior of new heterocyclic ring systems , Angewandte Chemie 70 (1958) 667-683.
- ↑ Jürgen Martens, Heribert Offermanns and Paul Scherberich: A simple synthesis of racemic cysteine , Angewandte Chemie 93 (1981) 680; Angewandte Chemie International Edition 20 668 (1981).
- ↑ K Gewald; Schinke, E .; Böttcher, H. Chemischeberichte 99, 1966, pp. 94-100.
- ↑ B. Kirste, FU Berlin: Substitution reactions on aromatics
- ↑ DT Davies: Basic Texts Chemistry: Aromatic Heterocyclen. 1st edition. Wiley-VCH, Weinheim, 1995, ISBN 3-527-29289-6 , pp. 37-39.
- ↑ KPC Vollhardt, NE Schore: Organic Chemistry 3rd Edition. Wiley-VCH, 2000, ISBN 3-527-29819-3 , pp. 1249-1250.
- ^ Author collective: Organikum. 21st edition. Wiley-VCH, 2001, ISBN 3-527-29985-8 , p. 395.
- ^ FW Bergstrom, HG Sturz, HW Tracy: The Use of the Fused Eutectic of Sodium amide and Potassium amide in Organic Synthesis. In: J. Org. Chem. 11, 1946, pp. 239-246; doi: 10.1021 / jo01173a005 .
- ^ JM Steward, CH Burnside in: Reactions of trimethylenesulfide with chlorine and bromine. In: J. Am. Chem. Soc. 1953, pp. 243-244; doi: 10.1021 / ja01097a517 .
- ↑ H. Beyer, W. Walter: Textbook of organic chemistry. 23rd edition. S. Hirzel Verlag, Stuttgart 1998, ISBN 3-7776-0808-4 , p. 332.
- ^ Author collective: Organikum. 21st edition. Wiley-VCH, 2001, ISBN 3-527-29985-8 , pp. 302-303.
- ↑ J. Schreiber, D. Felix, A. Eschenmoser, M. Winter, F. Gautschi, KH Schulte-Elte, E. Sundt, G. Ohloff, J. Kalovoda, H. Kaufmann, P. Wieland, G. Anner: The synthesis of acetylene-carbonyl compounds by fragmentation of alpha, beta-epoxy-ketones with p-toluenesulfonylhydrazine. In: Helv. Chim. Acta 50, 1967, pp. 2101-2108; doi: 10.1002 / hlca.19670500747 .
- ↑ D. Felix, J. Schreiber, G. Ohloff, A. Eschenmoser: α, β-epoxy ketone: alkynone fragmentation I: synthesis of exaltone and rac - muscone from cyclododecanone using synthetic methods. In: Helv. Chim. Acta 54, 1971, pp. 2896-2912; doi: 10.1002 / hlca.19710540855 .
- ↑ M. Baumann, IR Baxendale, SV Ley, N. Nikbin: An overview of the key routes to the best selling 5-membered ring heterocyclic pharmaceuticals. In: Beilstein J. Org. Chem. 7, 2011, pp. 442-495; doi: 10.3762 / bjoc.7.57 ( Open Access ).
- ↑ a b J. A. Joule, K. Mills: Heterocyclic Chemistry. 4th edition. 2000, Blackwell Oxford, ISBN 0-632-05453-0 , pp. 543-549.
- ↑ The sulfolane process (Engl.) ( Memento from 1 December 2010 at the Internet Archive ).
- ^ GA Paganini: Heterocycle-based electric conductors. In: Heterocycles 37, 1994, pp. 2069-2086.
- ↑ J. Roncali: Conjugated poly (thiophenes): synthesis, functionalization and application. In: Chem. Rev. 92, 1992, pp. 711-738; doi: 10.1021 / cr00012a009 .
- ^ J. Ferraris, DO Cowan, VV Walatka, JH Perlstein: Electron transfer in a new highly conducting donor-acceptor complex. In: J. Am. Chem. Soc. 95, 1973, pp. 948-949; doi: 10.1021 / ja00784a066 .
- ↑ F. Wudl: From organic metals to superconductors: managing conduction electrons in organic solids. In: Acc. Chem. Res. 17, 1984, pp. 227-232; doi: 10.1021 / ar00102a005 .
literature
- DT Davies: Basic Texts Chemistry: Aromatic Heterocycles. 1st edition. Wiley-VCH, Weinheim 1995, ISBN 3-527-29289-6 .
- JA Joule, K. Mills: Heterocyclic Chemistry. 4th edition. Blackwell Science, Oxford, 2000, ISBN 0-632-05453-0 .
- AR Katritzky, AF Pozharskii: Handbook of Heterocyclic Chemistry. 3. Edition. Elsevier, Amsterdam, 2004, ISBN 0-08-042989-0 .
- H. Beyer, W. Walter: Textbook of organic chemistry. 23rd edition. S. Hirzel Verlag, Stuttgart 1998, ISBN 3-7776-0808-4 , pp. 753-876.
Web links
- Lecture notes at the University of Tübingen ( Memento from April 12, 2015 in the Internet Archive ) (PDF file; 1.20 MB)
- Lecture notes University of Regensburg ( Memento from June 29, 2007 in the Internet Archive ) (PDF file; 1.97 MB)