Gewald reaction
The Gewald reaction is a name reaction in organic chemistry that was named after its discoverer, Karl Gewald (1930-2017). A ketone (or an aldehyde if R 2 = H) is reacted with an α-cyanoester in the presence of elemental sulfur and a base to form a substituted 2-amino thiophene .
Overview reaction
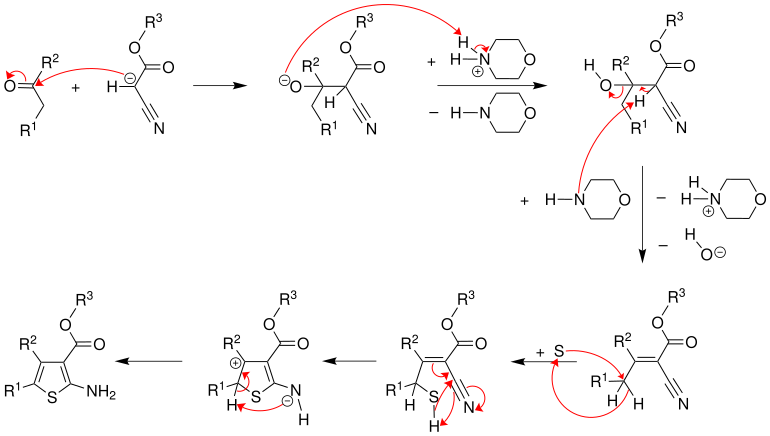
A ketone and an α-cyano ester to react with the base morpholine in a Knoevenagel condensation to a α- alkylidene nitrile, which with elemental sulfur to 2-amino- thiophen responding.
mechanism
The reaction mechanism could only be partially elucidated several years after the discovery of the reaction. In the first reaction step, the α-cyanoester is determined by the base morpholine deprotonated .
The resulting anion and ketone add up to an alkoxide , which in the further step by the protonated base to an alcohol protonated is. This is deprotonated to the stable intermediate product α- alkylidenenitrile .
The mechanism of the addition of elemental sulfur is unknown. It is assumed that the intermediate to be added plays a role. Cyclization and tautomerization then yield the 2-aminothiophene.
Supplying energy with microwaves sometimes shortens the reaction times while increasing the yield.
variants
In a modification of the Gewald reaction, 3-acetyl-2-aminothiophene is obtained when dithiane [an adduct of sulfur and acetone (R = CH 3 )] is reacted with freshly prepared cyanoacetone :
Individual evidence
- ^ John A. Joule, Keith Mills: Heterocyclic Chemistry , John Wiley & Sons, 5th Edition (2010), p. 340, ISBN 978-1-405-13300-5 .
- ↑ Bradford P. Mundy, Michael G. Ellerd, Frank G. Favaloro, Jr .: Name Reactions and Reagents in Organic Synthesis , John Wiley & Sons, 2nd Edition (2005) p. 306, ISBN 0-471-22854-0 .
- ↑ Christopher Hume: Applications of Multicomponent Reactions in Drug Discovery - Lead Generation to Process Development , pp. 311–341, there pp. 332–334, In Jieping Zhu, Huges Bienaymé: Multicomponent Reactions , Wiles-VCH Verlag, 2005, ISBN 978 -3-527-30806-4 .
- ↑ Gewald, K .; Schinke, E .; Böttcher, H. Chemical Reports 1966 , 99 , 94-100.
- ↑ Sabnis, RW Sulfur Reports 1994 , 16 , 1-17.
- ↑ Sabnis, RW; Rangnekar, DW; Sonawane, ND J. Heterocyclic Chem. 1999 , 36 , 333.
- ↑ K. Schwetlick: Organikum . 23rd edition. Wiley-VCH Verlag, Weinheim 2009, ISBN 978-3-527-32292-3 , pp. 434 .
- ↑ Sridhar, M .; Raoa, RM; Babaa, NHK; Kumbhare, RM Tetrahedron Lett. 2007 , 48 , 3171-3172, ( doi : 10.1016 / j.tetlet.2007.03.052 ).
- ↑ Gernot A. Eller, Wolfgang Holzer Molecules 2006 , 11, 371–376 Online article (PDF; 59 kB).