Morpholine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Morpholine ( IUPAC ) | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 4 H 9 NO | |||||||||||||||
| Brief description |
colorless liquid with an ammonia-like odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 87.12 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.00 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
−5 ° C |
|||||||||||||||
| boiling point |
129 ° C |
|||||||||||||||
| Vapor pressure |
|
|||||||||||||||
| pK s value |
8.36 |
|||||||||||||||
| solubility |
completely miscible with water |
|||||||||||||||
| Refractive index |
1.454 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
DFG / Switzerland: 10 ml m −3 or 36 mg m −3 |
|||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Morpholine is a heterocyclic organic compound that is liquid at room temperature and has the empirical formula C 4 H 9 NO. The saturated six-membered ring contains both an ether group and a secondary amine group; according to the Hantzsch-Widman system , it is therefore a hydrogenated (and therefore saturated) oxazine or an oxazinane .
Extraction and presentation
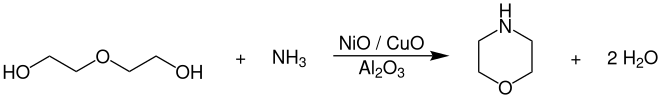
For large-scale production of morpholine are employed diethylene glycol with ammonia at temperatures of 130-240 ° C and pressures of 150-240 bar in the presence of nickel , copper and cobalt oxide - catalysts which on alumina (Al 2 O 3 are supported), around.
The complete reaction takes place in the liquid phase in a continuous tube or tube bundle reactor . The catalyst is arranged as a fixed bed and the reaction is preferably carried out in the trickle mode. The product is purified and worked up by multi-stage distillation in rectification columns .
Morpholine can also be obtained from diethanolamine or from bis (2-chloroethyl) ether . However, these processes are of no industrial importance.
properties
Physical Properties
Morpholine is a flammable, hygroscopic , colorless liquid with an amine-like odor. It reacts slightly alkaline with water. The boiling point at normal pressure is 128.95 ° C. According to Antoine, the vapor pressure function results from ln (P) = A− (B / (T + C)) (P in kPa, T in K) with A = 14.5733, B = 3384.26 and C = −61.453 im Temperature range from 308 to 393 K.
Safety-related parameters
Morpholine forms highly flammable vapor-air mixtures. The compound has a flash point of 31 ° C. The explosion range is between 1.8% by volume (64 g / m 3 ) as the lower explosion limit (LEL) and 15.2% by volume (550 g / m 3 ) as the upper explosion limit (UEL). The limit gap width was determined to be 0.92 mm. This results in an assignment to explosion group IIA. The ignition temperature is 275 ° C. The substance therefore falls into temperature class T3.
safety instructions
Morpholine is harmful if inhaled, swallowed and in contact with the skin. It causes chemical burns. Inhalation of morpholine leads to pulmonary edema and subsequent liver and kidney damage. With nitrosating compounds (e.g. nitrites , nitrogen oxides ), nitrosamines can form, which can be carcinogenic.
Morpholine has an LD 50 of 500 mg kg −1 (dermal, rabbit) and 1910 mg kg −1 (oral, rat).
use
- Additive to water and steam systems as a corrosion inhibitor and to adjust the pH value
- Manufacture of vulcanization aids for the rubber and rubber industry
- Addition when processing concrete
- Additive to waxes used to treat the surfaces of fresh fruit. This use is not permitted in the EU countries, but z. B. in the USA, Canada and South America.
- Production of the drugs Bufexamac , Dextromoramid , Doxapram , Emorfazon , Fenclofenac , Folescutol , Fomocain , Molindon , Moracizin , Morinamid , Naproxen , Sulmetocin , Timolol and Trimetotin as well as the PI3K inhibitor GDC-0941
- In organic synthesis as a weak amine base
- Especially for the synthesis of phenylacetic acid derivatives according to the Willgerodt-Kindler process
- One class of fungicides ( aldimorph , dodemorph , fenpropimorph , tridemorph ) has morpholine as a common structural motif
Individual evidence
- ↑ a b c d e f g h i j k l m n Entry on morpholine in the GESTIS substance database of the IFA , accessed on October 25, 2019(JavaScript required) .
- ^ The Evans Group: pKa's of Nitrogen Acids. (PDF) In: Chemistry and Chemical Biology. Harvard University, November 4, 2005, accessed August 23, 2019 .
- ↑ a b Morpholine data sheet from Sigma-Aldrich , accessed on April 11, 2011 ( PDF ).
- ↑ Entry on Morpholine in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers and / or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values , accessed on August 20, 2019.
- ↑ Data sheet morpholine (PDF) from Merck , accessed on February 27, 2010.
- ↑ a b c d e entry on morpholine. In: Römpp Online . Georg Thieme Verlag, accessed on May 27, 2020.
- ↑ a b Patent WO2007036496 : Process for the production of aminodiglycol (ADG) and morpholine. Published on April 5, 2007 , applicant: BASF AG, inventors: Bram Willem Hoffer, Holger Evers, Petr Kubanek, Till Gerlach, Johann-Peter Melder, Frank Funke, Matthias Frauenkron, Helmut Schmidtke.
- ^ Siegfried Hauptmann: Organische Chemie , Verlag Harry Deutsch, Thun 1985, ISBN 3-87144-902-4 , p. 602.
- ↑ Sovova, M .; Boublik, T .: Liquid-vapor equilibrium. Part C. Vapor-liquid equilibrium in the water (1) -morpholine (2) system at the pressures of 50 and 75 kPa in Collect. Czech. Chem. Commun. 51 (1986) 1899.
- ↑ Ming-Jer Lee, Chang-Ching Su, Ho-mu Lin: Vapor Pressures of Morpholine, Diethyl Methylmalonate, and Five Glycol Ethers at Temperatures up to 473.15 K in J. Chem. Eng. Data 50 (2005) 1535-1538, doi: 10.1021 / je049627d .
- ↑ a b c d E. Brandes, W. Möller: Safety-related parameters. Volume 1: Flammable Liquids and Gases. Wirtschaftsverlag NW - Verlag für neue Wissenschaft, Bremerhaven 2003.
- ↑ European Parliament: Subject: import ban on Chilean apples , Parliamentary question by Member of Parliament Filip Kaczmarek of 24 January 2011.
- ^ Axel Kleemann , Jürgen Engel, Bernd Kutscher and Dieter Reichert: Pharmaceutical Substances , 4th edition (2000) 2 volumes published by Thieme-Verlag Stuttgart, ISBN 978-1-58890-031-9 ; online since 2003 with biannual additions and updates.
- ^ Paul Workman, Paul A. Clarke, Florence I. Raynaud, Rob LM van Montfort: Drugging the PI3 Kinome: From Chemical Tools to Drugs in the Clinic . In: Cancer Research . tape 70 , no. 6 , March 15, 2010, p. 2146-2157 , doi : 10.1158 / 0008-5472.CAN-09-4355 ( PDF ).
- ^ Clemens Lamberth: Bioactive Heterocyclic Compound Classes . Ed .: Clemens Lamberth, Jürgen Dinges. Wiley-VCH, 2012, ISBN 978-3-527-66441-2 , Morpholine Fungicides for the Treatment of Powdery Mildew, p. 119-127 , doi : 10.1002 / 9783527664412.ch10 .