Nickel (II) oxide
| Crystal structure | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
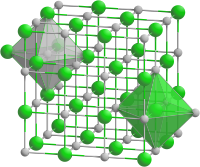
|
|||||||||||||||||||
| __ Ni 2+ __ O 2− | |||||||||||||||||||
| Crystal system | |||||||||||||||||||
| Lattice parameters |
a = 416.8 pm |
||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Nickel (II) oxide | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Ratio formula | NOK | ||||||||||||||||||
| Brief description |
odorless, dark green to black powder dark |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 74.69 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
6.72 g cm −3 |
||||||||||||||||||
| Melting point |
1984 ° C |
||||||||||||||||||
| solubility |
practically insoluble in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
no value given as it is carcinogenic |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Nickel (II) oxide is a chemical compound of the metal nickel that belongs to the group of oxides .
Occurrence
Of course, nickel (II) oxide occurs as a mineral bunsenite .
Extraction and presentation
It is obtained by intensive calcination of the nitrate (Ni (NO 3 ) 2 ) or carbonate (NiCO 3 ) or by oxidation of red nickel pebbles (NiAs) or pure nickel in the air.
properties
Nickel (II) oxide is a basic oxide. It is antiferromagnetic and is isotype with sodium chloride . The purest NiO is light yellow. A higher oxygen content gives the NiO a green to gray-green color. Darker to black products increasingly contain Ni 3+ . The nickel (II) oxide obtained at high temperatures is almost insoluble in acids and alkalis; the lower the preparation temperature, the more soluble it is, especially in hot nitric acid and ammonia water .
use
Nickel (II) oxide is used to manufacture enamel (as an adhesive), ceramics, glasses (as a coloring agent) and electrodes (for example for lithium-ion batteries ). It also serves as an anode material in fuel cells . It is also used as a catalyst for the hydrogenation of organic compounds. It is also an intermediate product for the production of pure nickel (reduction with carbon monoxide ).
safety instructions
Like many other nickel compounds, nickel (II) oxide is classified as a carcinogen.
Related links
- Nickel (III) oxide (nickel sesquioxide), Ni 2 O 3
- Nickel (IV) oxide (nickel dioxide), NiO 2
- Nickel (III) oxide hydroxide , NiO (OH)
- Nickel (II) hydroxide , Ni (OH) 2
Individual evidence
- ^ RW Cairns, E. Ott: X-Ray Studies of the System Nickel-Oxygen-Water. I. Nickelous Oxide and Hydroxide. In: Journal of the American Chemical Society , 55, 1933, pp. 527-533, doi : 10.1021 / ja01329a013 .
- ↑ a b c d e f g h Entry on nickel oxide in the GESTIS substance database of the IFA , accessed on December 7, 2019(JavaScript required) .
- ↑ Entry on nickel monoxide in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Georg Brauer (ed.) U. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume III, Ferdinand Enke, Stuttgart 1981, ISBN 3-432-87823-0 , p. 1689.
- ↑ Mila Schrader: Cast Iron Ovens and Stoves: A Historical Review ( Memento from March 17, 2009 in the Internet Archive )
- ^ Institute for Energy Research Jülich: The Jülich planar anode-supported high-temperature fuel cell (SOFC)
- ↑ M. Pehnt: Holistic balancing of fuel cells in energy and transport technology (PDF; 4.7 MB)


