Fomocaine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
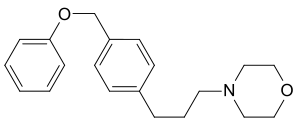
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Fomocaine | ||||||||||||||||||
| other names |
N - [3- (4-phenoxymethylphenyl) propyl] morpholine ( IUPAC ) |
||||||||||||||||||
| Molecular formula | C 20 H 25 NO 2 | ||||||||||||||||||
| Brief description |
colorless and odorless crystals |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| Mechanism of action |
unspecific membrane expansion on the sodium channel |
||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 311.4 g mol −1 | ||||||||||||||||||
| Melting point |
|
||||||||||||||||||
| pK s value |
Base: 7.1 |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Fomocaine is a morpholine - derivative that, as local anesthetic from the same class of Fomocaine, to 2003 as a surface anesthetic was used.
history
In the 1950s, the German pharmacist Herbert Oelschläger looked for stable and highly effective local anesthetics with low toxicity and found a useful component in the base morpholine. In 1967 fomocaine was introduced into therapy under the trade name Erbocain ® . In 1979 the hydrochloride was included in the German Medicines Codex , and in 1991 that of the base.
Fomocaine was used in ointments and gels in dermatology and in suppositories for the treatment of the haemorrhoidal symptom complex. However, there have been no more preparations on the market since 2003.
pharmacology
Fomocaine acts on voltage-dependent sodium channels in the cell membranes of the nerve cell, both by blocking and by being embedded in the membrane (unspecific membrane expansion). It is also possible to block calcium channels in which fomocaine has a similar affinity to e.g. B. has flecainide .
The systemic toxicity is low compared to other local anesthetics such as tetracaine and lidocaine . One reason for this is the high level of plasma protein binding .
Pharmacokinetics
Protein binding
95% of fomocaine is bound to plasma proteins, the majority of which is bound to serum albumin . The affinity for this is mediated via the two aromatic rings and the alkylene group.
Biotransformation
Fomocaine is almost completely metabolized, less than 5% is excreted unchanged. The most important metabolites are 4-OH-fomocaine, fomocaine- N -oxide and 4-OH-fomocaine- N -oxide, a total of 13 are known. The derivative 2-hydroxy-fomocaine still has a clearly local anesthetic effect in the cornea test.
Individual evidence
- ↑ a b c Dahse, Thomas: Synthesis, pharmacodynamics and biotransformation of the fomocaine derivative Oe 9000 , p. 23ff. Dissertation (PDF, 3.8 MB).
- ↑ a b c Knauthe, Sophie: In-vitro studies on the influence of the microsomal cytochrome P450 system in the rat liver and on possible pro- and / or antioxidant properties of new fomocaine derivatives and some fomocaine metabolites compared to fomocaine, procaine, lidocaine , dissertation, p. 16-17.
- ^ A b Hunnius Pharmaceutical Dictionary 9th Edition 2004, ISBN 3-11-017475-8 , pp. 603-604.
- ↑ Entry on fomocaine. In: Römpp Online . Georg Thieme Verlag, accessed on June 19, 2014.
- ↑ a b FOMOCAINE data sheet from Sigma-Aldrich , accessed on April 2, 2011 ( PDF ).
- ↑ Herbert Oelschläger: The Fomocaine from a chemical, pharmacokinetic and pharmacological point of view: Current status and outlook. In: Pharmacy in our time . Volume 29, No. 6, pp. 358-364, doi : 10.1002 / 1615-1003 (200012) 29: 6 <358 :: AID-PAUZ358> 3.0.CO; 2-3 .