Human albumin
| Human albumin | ||
|---|---|---|
|
||
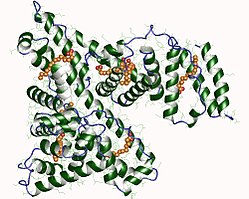
| Surface model of human albumin with lipid molecules according to PDB 1E7H | ||
|
Existing structure data: 1ao6 , 1bj5 , 1bke , 1bm0 , 1e78 , 1e7a , 1e7b , 1e7c , 1e7e , 1e7f , 1e7g , 1e7h , 1e7i , 1gni , 1gnj , 1h9z , 1ha2 , 1hk1 , 1hk2 , 1hk3 , 1hk4 , 1hk5 , 1n5u , 1o9x , 1tf0 , 1uor , 1ysx , 2bx8 , 2bxa , 2bxb , 2bxc , 2bxd , 2bxe , 2bxf , 2bxg , 2bxh , 2bxi , 2bxk , 2bxl , 2bxm , 2bxn , 2bxo , 2bxp , 2bxq , 2esg , 2i2z , 2i30 |
||
| Properties of human protein | ||
| Mass / length primary structure | 585 amino acids | |
| Isoforms | 2 | |
| Identifier | ||
| Gene name | ALB | |
| External IDs | ||
| Drug information | ||
| ATC code | B05 AA01 | |
| DrugBank | DB00062 | |
| Occurrence | ||
| Homology family | albumin | |
| Parent taxon | Amniotes | |
The human albumin is the human form of the albumin . It is a globular protein found in the blood. Albumin is used to maintain the oncotic pressure . Among other things, it is reduced in liver diseases. In healthy people, there is a concentration of 35 to 53 g / l in the serum. Mutations in ALB - gene are for a rare hereditary form of hyperthyroxinemia responsible. Very rarely the protein can be completely absent.
properties
Albumin has a molecular mass of about 66,470 Da and is elliptical in shape. It consists of 585 amino acids , many of which contain sulfur. It is soluble in water, the binding capacity for water is approx. 18 ml / g. The isoelectric point is pH 4.6. Albumin is an ampholyte ; H. In contrast to other colloids or crystalloids , it can bind both anions and cations reversibly.
Occurrence
On average, a person (70 kg) in a healthy state has 250-320 g of albumin. 40% of these are dissolved inside the blood vessels in the blood plasma , 60% outside the vessels in the tissue. In addition to albumin, there are other proteins, the globulins, in the blood. Although albumin is the smaller of the two plasma proteins , they are in the majority with 60% (3.5–4.5 g / dl) in terms of quantity, globulins only make up 40%.
biosynthesis
Albumin is the gene product of the ALB - gene , which on chromosome 4 gene locus is located q13.3. The synthesis of albumin takes place in the liver with an average synthesis rate of 0.2 g albumin / kg body weight and day. The preproalbumin produced by the liver cells ( hepatocytes ) is post-translationally modified in the rough endoplasmic reticulum (rER) . The removal of amino acids at the N-terminus produces proalbumin. The trial albumin is ultimately converted to albumin in the Golgi vesicles . The breakdown takes place via the kidneys , the gastrointestinal tract and in the tissue cells of the liver.
In 2011, the Chinese researcher Daichang Yang succeeded in producing human albumin using genetically modified rice plants .
Biological function
Maintenance of the colloid osmotic pressure
By binding water to itself in the blood vessels, albumin prevents it from escaping into the intercellular spaces and thus into the tissue . Since albumins are responsible for 80 percent of the colloid osmotic pressure in the plasma, their deficiency automatically reduces the pressure and leads to edema (water retention in the tissue).
Transport of water-insoluble substances in the blood
Hydrophobic substances such as fatty acids , bilirubin , trace elements , certain vitamins , hormones , cations (Mg 2+ , Ca 2+ ) and drugs could not be transported in the blood without binding to albumins. A single albumin molecule can reversibly bind up to 20-25 bilirubin molecules, 9 stearic acid molecules or 5 salicylic acid molecules.
Contribution to the buffer capacity of the blood
Due to their ampholytic properties, albumins are not able to
H + ions both absorb and release and can thus have a stabilizing effect on the pH value of the blood. However, the albumin has a much smaller proportion of the buffering capacity of the blood than, for example, hydrogen carbonate and hemoglobin .
Disturbances in the albumin balance
Albumin is produced in the liver , which is important in various diseases. In cirrhosis of the liver (e.g. due to chronic alcohol abuse ), the organ's ability to synthesize albumin is restricted ( hypalbuminemia ), which leads to edema and ascites due to the reduced colloid osmotic pressure of the blood plasma . The situation is similar in the case of chronic malnutrition , as the lack of protein and amino acid intake also reduces the formation of albumin, and the serum albumin alongside other protein reserves, such as B. muscle tissue is broken down. Hypoalbuminemia with albuminuria during pregnancy is a non-pathological loss of albumin.
Use in vaccines
Human albumin is used as a stabilizer in some vaccines (e.g. rabies , TBE or rubella vaccines ). No allergic reactions are known.
See also
Individual evidence
- ↑ human albumin. In: Online Mendelian Inheritance in Man . (English)
- ↑ Orphanet: Familial analbuminemia
- ↑ genenames.org: ALB , accessed February 21, 2009.
- ↑ Blood protein from rice grains
- ↑ patented method
- ↑ J. Liese and M. Prelog: Vaccinations and allergies . In: Heinz Spiess, Ulrich Heininger, Wolfgang Jilg (Eds.): Impfkompendium . 8th edition. Georg Thieme Verlag, 2015, ISBN 978-3-13-498908-3 , p. 312 .
