Aldimorph
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||
| Basic structural formula (stereocenters are marked with an * ) | ||||||||||
| General | ||||||||||
| Surname | Aldimorph | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 18 H 37 NO | |||||||||
| Brief description |
colorless liquid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 283.49 g mol −1 | |||||||||
| Physical state |
liquid |
|||||||||
| Melting point |
-27 to -19 ° C |
|||||||||
| boiling point |
110-140 ° C (10 Pa ) |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| Toxicological data | ||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Aldimorph is a chemical compound from the group of morpholines . It was brought onto the market by Fahlberg-List in 1980 as a fungicide and is no longer approved.
Stereoisomers
The active ingredient contains two similarly substituted stereocenters on the heterocyclic ring, consequently there are three stereoisomers:
- (2 R , 6 R ) -4-dodecyl-2,6-dimethylmorpholine,
- (2 S , 6 S ) -4-dodecyl-2,6-dimethylmorpholine and
- meso -4-dodecyl-2,6-dimethylmorpholine.
| Aldimorph |
|---|
 (2 R , 6 R ) isomer |
 (2 S , 6 S ) isomer |
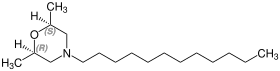 meso shape |
composition
In addition to the main component, aldimorph consists of various 4- N -alkyl-2,6-dimethylmorpholines.
use
Under the trade name Falimorph , it was used against powdery mildew in grain cultivation.
effect
Straight-chain N- alkyl-2,6-dimethylmorphine have a high fungicidal toxicity with chain lengths of C 10 to C 14 . Although they are less effective than the branched compounds, they are more phytotoxic.
Admission
No plant protection products containing this active ingredient are permitted in the EU or Switzerland .
Individual evidence
- ↑ a b c d e f Entry on Aldimorph. In: Römpp Online . Georg Thieme Verlag, accessed on November 20, 2014.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ H. Dobe, K. Sieber, A. Jumar: Studies on the metabolism of aldimorph in seedlings of Hordeum distichon. In: Biochemistry and Physiology of Plants. 181, 1986, pp. 91-102, doi : 10.1016 / S0015-3796 (86) 80077-4 .
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on aldimorph in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on March 26, 2016.