Fulval
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||
| General | |||||||||||||
| Surname | Fulval | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 10 H 8 | ||||||||||||
| Brief description |
orange liquid |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 128.17 g mol −1 | ||||||||||||
| Physical state |
liquid |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Fulvalen is a chemical compound belonging to the cycloalkenes group . Formally it consists of two cyclopentadiene rings that are linked by a double bond . Fulvalen is isomeric to naphthalene and azulene .
presentation
The first attempt at the synthesis of fulvalene by oxidative coupling of cyclopentadienyl salts with iron (III) chloride led to the discovery of ferrocene .
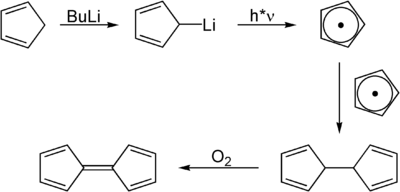
Fulvalene can be produced from the reaction of cyclopentadiene with butyllithium . A cyclopentadienyl radical is formed as an intermediate and dimerizes . This is then carried oxygen to fulvalene oxidized .
properties
Fulval is an orange liquid. The compound is unstable and easily dimerizes: in addition to the Diels-Alder reaction , which proceeds analogously to the dimerization of cyclopentadiene , it also undergoes [2 + 2] cycloadditions . Electron-poor perchlorfulvalene (C 10 Cl 8 ) is relatively stable . Tetrathiafulvalene (C 6 H 4 S 4 ) is an organic semiconductor .
Individual evidence
- ↑ a b A. G. Davies, JRM Giles, J. Lusztyk: The Electron Spin Resonance Spectrum of the Fulvalene Radical Anion . In: J. Chem. Soc. Perkin Trans. 2 1981 , 747-752.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ TJ Kealy, PL Pauson: A New Type of Organo-Iron Compound . In: Nature 1951 , 168 , 1039.
- ↑ A. Escher, W. Rutsch, M. Neuenschwander: Synthesis of pentafulvalene by oxidative coupling of cyclopentadienide using copper (II) chloride . In: Helv. Chim. Acta 1986 , 69 , 1644-1654. doi : 10.1002 / hlca.19860690719
- ^ V. Mark: Perchlorofulvalene In: Organic Syntheses . 46, 1966, p. 93, doi : 10.15227 / orgsyn.046.0093 ; Coll. Vol. 5, 1973, p. 901 ( PDF ).
- ^ Hans Beyer and Wolfgang Walter : Textbook of Organic Chemistry , 23rd edition, S. Hirzel Verlag, Stuttgart 1998, ISBN 3-7776-0808-4 , p. 425.