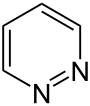
Pyridazine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Pyridazine | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 4 H 4 N 2 | |||||||||||||||
| Brief description |
colorless to yellow-brown liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 80.09 g · mol -1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.10535 g cm −3 (20.0 ° C) |
|||||||||||||||
| Melting point |
−8 ° C |
|||||||||||||||
| boiling point |
208 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.52311 (23.5 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
224.9 kJ / mol |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Pyridazine is a heterocyclic , aromatic organic compound . It consists of a six-membered ring that has two adjacent nitrogen atoms. The systematic name is 1,2-diazine , the compound has the empirical formula C 4 H 4 N 2 . Pyridazine belongs to the group of diazines and forms the basic structure of the pyridazines , which are isomeric to the pyrimidines and pyrazines .
properties
Pyridazine is a colorless to yellow-brown liquid that is miscible with water. Their hydrochloride is a yellow solid that melts at 161-163 ° C. Their monopicrate and chloro aurate are also yellow solids that decompose at 170 ° C and 110 ° C, respectively.
Pyridazine is a hückel aromatic compound that has six π electrons . Both nitrogen atoms also have a free electron pair each . This enables the binding of a proton and the formation of complexes with metals and metal ions .
Nitrogen atoms are more electronegative than carbon atoms , which is why, in contrast to benzene , the electron density in aromatics is reduced and also unevenly distributed. The highest electron density is found on the nitrogen atoms. Compared to pyridine , diazines have an additional electron-withdrawing nitrogen atom, which means that the electron density in the aromatic is even lower in pyridine. Due to the competition of the two nitrogen atoms to the available electron pyridazine has a lower basicity as pyridine to ( pK s values of conjugate acids : pyridine: 5.23, pyridazine: 2.24).
presentation
After the synthesis originally described by R. Marquis in 1901 and revised in 1942 by Hückel and Jahnentz, furan is nitrated with a mixture of nitric acid and acetic anhydride ; the nitroacetofuran obtained as an intermediate is immediately reacted further with hydrazine hydrate with a moderate yield of 20%.
Better yields are achieved when converting maleic acid derivatives . Maleic anhydride can thus be successfully subjected to hydrazinolysis. The resulting 1,2-dihydropyridazine-3,6-dione can be converted to pyridazine with up to 62% yield after chlorination with phosphorus oxychloride with H 2 -Pd / C.
The reaction of maleic dialdehyde with hydrazine also leads to pyridazine. Due to the unstable character of the dialdehyde, it is mostly released in situ from one of its corresponding diacetals. The yield of this method could be increased to over 80% in a BASF process through minor modifications of the original instructions (in particular a changed sequence of the work steps):
Individual evidence
- ↑ a b data sheet pyridazine from Acros, accessed on February 19, 2010.
- ^ A b c Walter Hückel, Walter Jahnentz: The association of pyrazole and pyridazine. In: Reports of the German Chemical Society. Volume 75, No. 12, 1942, pp. 1438-1446.
- ↑ a b Pyridazine data sheet from Sigma-Aldrich , accessed on April 22, 2011 ( PDF ).
- ^ A b c d e Raymond Nielson Castle: The Chemistry of Heterocyclic Compounds: Volume 28 Pyridazines . John Wiley & Sons, 1973, ISBN 978-0-471-38213-3 ( page 6 in Google Book Search).
- ^ Robert Cooley Elderfield: Heterocyclic Compounds: Six-membered heterocycles containing two hetero atoms and their benzo derivatives . Wiley, 1957, pp. 110 .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-25.
- ↑ a b J. A. Joule, K. Mills: Heterocyclic Chemistry , pp. 249-288, 5th edition, p. 7, Blackwell Publishing, Chichester, 2010, ISBN 1-4051-3300-7 .
- ↑ a b c D. T. Davies: Basistexte Chemie: Aromatic Heterocyclen, 1st edition, Wiley-VCH, Weinheim 1995, ISBN 3-527-29289-6 , p. 73.
- ^ R. Marquis: Compt. rend. Acad. Sciences 1901 , 132: 140.
- ↑ RH Mizzoni and Paul. E. Spoerri, "Synthesis in the Pyridazine Series. I. Pyridazine and 3,6-Dichloropyridazine", Journal of the American Chemical Society 1951 , 73: 1873-1874.
- ^ CM Atkinson and CJ Sharpe: "The Phenylation of Some Diazines." Journal of the Chemical Society 1959 : 3040-3046.
- ↑ Dong-bo Fan, Li-yan Dai, Xiao-zhong Wang and Ying-qi Chen: "Improved Synthesis Process of Pyridazine", Huaxue Shijie (Chemical World) 2007 , 48 (9): 549-552.
- ↑ A. Wohl and E. Bernreuther: "About Derivate des Asparaginaldehyds. I.", Liebigs Annalen der Chemie 1930 , 481: 1-29.
- ↑ M. Brüggemann and K. Ebel (BASF AG): "Process for the production of pyridazine", DE 102005029094 A1 2007 .


