Hydrazine
| Structural formula | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
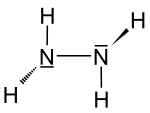
|
|||||||||||
| General | |||||||||||
| Surname | Hydrazine | ||||||||||
| other names |
|
||||||||||
| Molecular formula | N 2 H 4 | ||||||||||
| Brief description |
colorless, clear liquid |
||||||||||
| External identifiers / databases | |||||||||||
|
|||||||||||
| properties | |||||||||||
| Molar mass | 32.05 g mol −1 | ||||||||||
| Physical state |
liquid |
||||||||||
| density |
1.01 g cm −3 (20 ° C) or 1.00 g cm −3 (25 ° C) |
||||||||||
| Melting point |
|
||||||||||
| boiling point |
|
||||||||||
| Vapor pressure |
21 h Pa (20 ° C) |
||||||||||
| pK s value |
|
||||||||||
| solubility |
miscible with water |
||||||||||
| Refractive index |
1.47 (20 ° C) |
||||||||||
| safety instructions | |||||||||||
|
|||||||||||
| Authorization procedure under REACH |
particularly worrying : carcinogenic ( CMR ) |
||||||||||
| MAK |
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||
Hydrazine is a very toxic inorganic chemical compound made from nitrogen and hydrogen with the empirical formula N 2 H 4 . It is a colorless, oily liquid that smells like ammonia and smokes in air. Hydrazine burns with a barely visible flame. It is usually sold as an aqueous solution or as hydrazine hydrate (H 2 N − NH 2 · H 2 O).
Manufacturing
Technically
Technically, there are several ways to synthesize hydrazine:
- Raschig synthesis : oxidation of ammonia (NH 3 ) with sodium hypochlorite .
- Ammonia solution and hypochlorite ions react quickly to form chloramine and hydroxide ions .
- Excess ammonia gas is pressed into the solution at high pressure, which heats up to approx. 400 K, and the reaction continues to form hydrazine.
- Bayer process: Oxidation of ammonia with sodium hypochlorite in the presence of acetone .
- With acetone, hydrazine forms a ketazine, the condensation product of a ketone with hydrazine.
- The Ketazine can be at 8-12 bar and 180 ° C to hydrazine hydrolyze .
- Pechiney- Ugine-Kuhlmann process: The technically increasingly used process consists of the oxidation of ammonia with hydrogen peroxide (H 2 O 2 ) in the presence of methyl ethyl ketone as a ketazine generator and acetamide and sodium dihydrogen phosphate as activators .
- The ammonia-hydrogen peroxide mixture reacts with the ketone to form a ketazine and water .
- As in the Bayer process, the ketazine obtained can easily be converted into hydrazine by hydrolysis .
- The advantages over the Raschig and Bayer processes are lower energy consumption and the absence of chlorides .
In the laboratory
- By introducing chlorine into a 20% urea solution and then adding 20% sodium hydroxide solution . The yield is around 50%.
- The mechanistic procedure is the same as that of the Hofmann rearrangement : First, formation of the amidate ion by De protonation , then electrophilic halogenation of the amidate ion, followed by the second deprotonation. The chloramidation spontaneously breaks down to acyl nitrene and a chloride ion. The acyl nitrene rearranges to form isocyanate , which, with the addition of water, produces the inconsistent carbamic acid , which then breaks down to carbon dioxide and hydrazine. The resulting carbon dioxide is absorbed by the sodium hydroxide solution with the formation of sodium carbonate .
- Urea , chlorine and sodium hydroxide solution react to form hydrazine, sodium carbonate and sodium chloride .
- The monohydrate can also be obtained from dry hydrazinium sulfate and potassium hydroxide by adding water and subsequent distillation in a yield of 25% of theory:
- 99.5% anhydrous hydrazine is formed in the fractional distillation of the monohydrate with sodium hydroxide in a stream of nitrogen.
- Another way of producing dry hydrazine is to release hydrazine from hydrazinium salts by adding the stronger base ammonia as an acid binder:
properties
Pure hydrazine can disproportionate explosively to ammonia and nitrogen when heated .
Concentrated solutions are highly explosive in connection with oxidizing agents , sometimes hypergolic . Hydrazine can also be catalytically decomposed; this is used in technology, e.g. B. in correction or emergency engines.
The compound forms an azeotrope with water with a hydrazine content of 58.5%, which boils at 120.5 ° C.
Acid / base behavior
Hydrazine is a divalent base ( pK b1 = 6.07; pK b2 = 15), but weaker than ammonia pK b = 4.75). As a divalent base it reacts with acids to form two series of hydrazinium salts (name analogous to ammonium ) with the general composition [H 2 N − NH 3 ] + X - and [H 3 N − NH 3 ] 2+ 2X - . With hydrochloric acid, hydrazinium monochloride ([H 2 N − NH 3 ] Cl) and hydrazinium dichloride ([H 3 N − NH 3 ] Cl 2 ) are formed. With sulfuric acid hydrazinium sulfate ([H 3 N − NH 3 ] SO 4 ) and dihydrazinium sulfate ([H 2 N-NH 3 ] 2 SO 4 .
Compared to very strong bases (pK b ≪ 0), hydrazine also functions as an acid. By reacting sodium hydride or sodium amide with hydrazine, sodium hydrazide which is extremely sensitive to oxidation and in which hydrazide anions are present (N 2 H 3 - ) can be obtained. Conversely, hydrazide ions react practically completely with water to form hydroxide ions and hydrazine.
use
Energy supplier (fuel, propulsion means)
Rocket fuel
Due to its highly reactive properties, hydrazine is mainly used as a rocket fuel , which forms a hypergolic fuel combination with the oxidizers dinitrogen tetroxide or nitric acid . Hydrazine is not only used pure, but also mixed together with 1,1-dimethylhydrazine with the oxidizers mentioned above. Known mixtures with different concentrations of the two components are Aerozin 50 and UH 25 .
Fuel cells
Hydrazine can be used in suitable fuel cells, the hydrazine fuel cells , to generate electrochemical electricity. The storage of the liquid hydrazine hydrate can, compared to the gaseous hydrogen, take place in tanks of any shape, since these can be designed for lower pressures. Due to the toxicity of hydrazine, the use of hydrazine fuel cells is only conceivable in special areas such as space travel or the military .
Mono propellants for thrusters, emergency power units and surfacing systems
Hydrazine is used in correction engines, where it is catalytically decomposed in a strongly exothermic reaction into gaseous nitrogen and hydrogen, for example also in the Voyager probes . According to company information from 2018, more than 500 hydrazine-powered thrusters are working in space, and they are also used in Ariane 5 .
In single-jet aircraft such as the F-16 , hydrazine and a catalytic decomposer are used as an emergency power generator.
In submarines , e.g. B. in the submarine class 214 , a hydrazine decomposer is used as an integral component of a rescue system called RESUS (REscue system for SUbmarineS) and which, with the help of nitrogen pressure, enables the submarine to emerge even when other systems Have failed.
chemistry
Corrosion inhibitor
Diluted hydrazine solutions are also used as reagents in the laboratory and for deoxygenation (liberation of oxygen) from boiler feed water in steam power plants. It is used both for removing the residual oxygen after degassing the feed water, for protection against possible minor oxygen ingresses in the area of the condenser and for catalytic oxygen removal from the make-up water. The advantage of hydrazine is that only nitrogen and water are produced in this reaction. In addition to deoxygenation, the pH value in the water-steam cycle is also increased .
Reducing agent
In chemical synthesis , hydrazine is mainly used as a strong nucleophile (so-called alpha effect ) and as a reducing agent for carbonyl groups ( Wolff-Kishner reaction ) or as a hydrogen source in catalytic hydrogenation .
Environmental hazards

Hydrazine is used as a storable fuel in many rockets, satellites and spacecraft. This can create a significant environmental hazard if a missile launch fails. If a satellite has already reached orbit , due to the high speed of at least eight kilometers per second and the unfavorable aerodynamic conditions in the case of the spherical tanks, it is practically impossible for them to hit the ground, as they burn up due to the high kinetic energy in the atmosphere . The hydrazine decomposes in the process.
No hydrazine contamination was found in the Challenger disaster . After a successful shuttle landing, one of the first safety measures was always to examine the stationary orbiter for leaking hydrazine. Only if this test was negative were other auxiliary vehicles, e.g. for cooling, allowed to approach the shuttle.
During the 2003 Columbia disaster, media warned of possible hydrazine contamination by NASA, and in fact, a largely intact Columbia hydrazine tank was found in 2011.
The shooting down of the American USA-193 satellite was justified in the media with the threat posed by the hydrazine on board.
safety instructions
Hydrazine is very toxic, carcinogenic in animal experiments and has a highly toxic effect on aquatic organisms. Hydrazine is also absorbed through the skin. Hydrazine has an acceptable concentration of 1.7 ppb and a tolerance concentration of 17 ppb according to TRGS 910.
The use of hydrazine in water-steam systems (steam boiler systems and district heating systems) has been subject to strict regulations since 1991, which are defined in TRGS 608. So is z. B. Direct DHW heating in district heating systems that are conditioned with hydrazine is not permitted, but a two-circuit system must be installed.
Hydrazine has been included in the SVHC candidate list (list of substances of very high concern) since June 2011 because of a suspected carcinogenic effect . Initially, this only has an impact on specific information requirements in the supply chain.
See also
Individual evidence
- ↑ a b c d e f g h i j k l m n Entry on hydrazine in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ^ Charles E. Mortimer: Chemistry - The basic knowledge of chemistry . Thieme 2003, ISBN 3-13-484308-0 .
- ^ CRC Handbook of Chemistry and Physics, Ed. DR Lide, CRC Press, Boca Raton, FL, 2005.
- ↑ a b Datasheet Hydrazine, anhydrous at Sigma-Aldrich , accessed on May 12, 2016 ( PDF ).
- ↑ Entry on hydrazine in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Entry in the SVHC list of the European Chemicals Agency , accessed on July 16, 2014.
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 302-01-2 or hydrazine ), accessed on November 2, 2015.
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 .
- ↑ G. Brauer (Ed.): Handbook of Preparative Inorganic Chemistry , 2nd ed., Vol. 1, Academic Press 1963, pp. 469-472.
- ↑ Hydrazine has a dynamic viscosity of 0.9 · 10 −3 Pa · s.
- ↑ Entry on hydrazine. In: Römpp Online . Georg Thieme Verlag, accessed on June 14, 2014.
- ^ Fourth movement of the symphony . In: Der Spiegel . No. 35/1989 , August 28, 1989, ISSN 0038-7452 , p. 190-195 ( online ).
- ↑ 20N Monopropellant Hydrazine Thruster. ArianeGroup , accessed February 5, 2020 .
- ↑ Chemical monopropellant thruster Family. In: Hydrazine Thrusters. Ariane Group, 2018, accessed June 22, 2019 .
- ↑ Help with aircraft accidents. (PDF) (No longer available online.) In: Landesfeuerwehrverband Brandenburg e. V. General Aviation Safety in the Bundeswehr, p. 10 , formerly in the original ; accessed on July 6, 2013 (2nd edition 2007). ( Page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice.
- ↑ https://aviation.stackexchange.com/questions/23417/why-is-hydrazine-used-to-power-the-f-16s-epu#23421
- ↑ RESUS - Rescue Systems for Submarines. In: Orbital Propulsion Center, Lampoldshausen, Germany> Submarine Recovery and Rescue Systems. ArianeGroup GmbH, Taufkirchen, accessed on June 22, 2019 (English).
- ↑ K. Hancke, S. Wilhelm: Water treatment: chemistry and chemical process engineering. Springer, 2003, ISBN 3-540-06848-1 , p. 249.
- ↑ F. Zymalkokowski: Catalytic Hydrogenation , Ferdinand Enke Verlag, Stuttgart 1965.
- ↑ Byron Harris: NASA says object found in East Texas lake is from doomed shuttle. (No longer available online.) WFAA.com, Aug 2, 2011, archived from the original on March 10, 2016 ; accessed on March 10, 2016 (English). Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ BAuA: Technical rule for hazardous substances 608. November 13, 2001, accessed on January 5, 2013 .



















