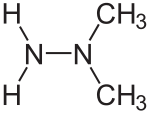
1,1-dimethylhydrazine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 1,1-dimethylhydrazine | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 2 H 8 N 2 | |||||||||||||||
| Brief description |
colorless, amine-like smelling liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 60.10 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.78 g cm −3 |
|||||||||||||||
| Melting point |
−58 ° C |
|||||||||||||||
| boiling point |
63 ° C |
|||||||||||||||
| Vapor pressure |
164 h Pa (20 ° C) |
|||||||||||||||
| solubility |
completely miscible with water |
|||||||||||||||
| Refractive index |
1.4075 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
Switzerland: 0.5 ml m −3 or 1.2 mg m −3 |
|||||||||||||||
| Toxicological data | ||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
48.9 kJ / mol |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
1,1-dimethylhydrazine (dec. UDMH for U nsymmetrisches D i m ethyl h ydrazin) is the two-fold at a N-atom methylated hydrazine. In contrast to the symmetrical dimethylhydrazine ( 1,2-dimethylhydrazine ), here both methyl groups are bonded to the same nitrogen atom.
history
1,1-Dimethylhydrazine has been used as a rocket fuel since the 1950s because of its shelf life . Today nitrous oxide is mostly used as the oxidizing agent ; before that, nitric acid was often used.
Presentation and extraction
The production of 1,1-dimethylhydrazine is analogous to the Raschig synthesis from chloramine and dimethylamine .
A second synthetic route starts from acetic hydrazide , which is reductively alkylated with formaldehyde and hydrogen in the first step . In the second step, hydrolysis gives the target compound.
properties
UDMH is a colorless, fish-like smelling liquid that boils at 63 ° C at normal pressure . The heat of vaporization at the boiling point is 32.55 kJ mol −1 . The vapor pressure function results according to Antoine according to log 10 (P) = A− (B / (T + C)) (P in bar, T in K) with A = 4.71316, B = 1388.51 and C = −40.613 in the temperature range from 237.74 to 293.08 K. The vapors of 1,1-dimethylhydrazine can irritate the skin and the mucous membranes (eyes, respiratory tract) or burn them in the event of severe exposure. At 20 ° C it has a dynamic viscosity of 0.56 · 10 −3 Pa · s.
The heat of decomposition determined by DSC is −69 kJ · mol −1 or −1150 kJ · kg −1 .
Because of its low vapor pressure and its high reactivity (especially with respect to ozone ), it is not to be expected that it will be distributed over a wide area when it enters the environment; there is rapid degradation.
Safety-related parameters
1,1-Dimethylhydrazine forms highly flammable vapor-air mixtures. The compound has a flash point of −18 ° C. The explosion range is between 2% by volume (50 g / m 3 ) as the lower explosion limit (LEL) and 20% by volume (490 g / m 3 ) as the upper explosion limit (UEL).) The limit gap width was 0.85 mm determined. This results in an assignment to explosion group IIB. The ignition temperature is 240 ° C. The substance therefore falls into temperature class T3.
use
- For the production of dyes , pharmaceuticals and man-made fibers.
- As a gas absorbent for carbon dioxide and sulfur dioxide .
- 1,1-Dimethylhydrazine is the flammable component ( heptyl , Russian) of liquid hypergolic rocket propellants when used together with the oxidizers dinitrogen tetroxide ( amyl , Russian) or RFNA (fuming nitric acid, AK-27I or Mélange , Russian). The Proton launch vehicle uses 1,1-dimethylhydrazine as fuel for several or all stages, which in the event of false starts leads to contamination in the crash area. In the first Gulf War used Soviet Scud missiles contained 1000 kg each UDMH and 3500 kg fuming.
- 1,1-Dimethylhydrazine is not only used pure, but also mixed with hydrazine as a rocket fuel. Known mixtures with different concentrations of the two components are Aerozin 50 and UH 25 .
- In the fall of 2017, UDMH was adopted by experts as a rocket fuel in North Korea's nuclear program.
- At the moment 1,1-dimethylhydrazine is only produced in large quantities in Russia and the People's Republic of China .
physiology
1,1-Dimethylhydrazine is easily absorbed through the skin and has been shown to be carcinogenic in animal experiments .
Individual evidence
- ↑ a b c d e f g h i j k l m entry on N, N-dimethylhydrazine in the GESTIS substance database of the IFA , accessed on October 24, 2018(JavaScript required) .
- ↑ Data sheet 1,1-dimethylhydrazine from Sigma-Aldrich , accessed on March 5, 2011 ( PDF ).
- ↑ Entry on N, N-dimethylhydrazine in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 57-14-7 or 1,1-dimethylhydrazine ), accessed on November 2, 2015.
- ↑ a b data sheet 1,1-dimethylhydrazine (PDF) from Merck , accessed on October 8, 2004.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-23.
- ↑ a b Schiermann, J.-P .; Bourdauducq, P .: Hydrazine , in: Ullmanns Enzyklopädie der Technischen Chemie , Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim 2005; doi : 10.1002 / 14356007.a13_177 .
- ↑ a b Majer, V .; Svoboda, V .: Enthalpies of Vaporization of Organic Compounds: A Critical Review and Data Compilation , Blackwell Scientific Publications, Oxford, 1985, 300.
- ↑ Aston, JG; Wood, JL; Zolki, TP: The thermodynamic properties and configuration of unsymmetrical dimethylhydrazine in J. Am. Chem. Soc. 75 (1953) 6202-6204, doi : 10.1021 / ja01120a027 .
- ↑ Grewer, T .; Klais, O .: Exothermic decomposition - investigations of the characteristic material properties , VDI-Verlag, series "Humanisierung des Arbeitsleben", Volume 84, Düsseldorf 1988, ISBN 3-18-400855-X , p. 9.
- ↑ a b c E. Brandes, W. Möller: Safety-related parameters. Volume 1: Flammable Liquids and Gases. Wirtschaftsverlag NW - Verlag für neue Wissenschaft, Bremerhaven 2003.
- ^ Rudolf Meyer: Explosivstoffe , 6th edition, VCH Verlagsgesellschaft, Weinheim 1985, ISBN 3-527-26297-0 , pp. 92-93.
- ^ Rudolf Meyer: Explosivstoffe , 6th edition, VCH Verlagsgesellschaft, Weinheim 1985, ISBN 3-527-26297-0 , p. 5.
- ↑ nytimes.com: The Rare, Potent Fuel Powering North Korea's Weapons . 17th September 2017.
- ^ Schmucker Robert & Schiller Markus: Missile Threat 2.0: Technical and Political Basics . Mittler Verlag, 2015, ISBN 3-8132-0956-3 , p. 84.







