Wolff-Kishner reaction
The Wolff-Kishner reaction or Wolff-Kishner reduction is a chemical reaction with the help of which it is possible to reduce aldehydes and ketones to alkanes . The reaction bears the name of its discoverer, the German chemist Ludwig Wolff and the Russian chemist Nikolai Kischner (1867–1935).
mechanism
In the reaction, the carbonyl compound is first reacted with hydrazine to form the hydrazone . Subsequently, without isolating the hydrazone, nitrogen is eliminated by strong bases (here hydroxide ion) and the alkane remains. The Wolff-Kishner reaction is complementary to the Clemmensen reduction , which is used in acids to reduce ketones and aldehydes. The Wolff-Kishner reaction is only suitable for base-stable compounds due to the harsh reaction conditions (high temperature, addition of solid KOHs).
Variant according to Huang Minlon
The variant developed by Huang Minlon , a Chinese chemist, leads to better yields by using a high-boiling solvent. The use of solid KOH in diethylene glycol has now completely replaced the use of potassium hydroxide solution.
The variant was discovered when a reaction caused the stopper of a flask to fly off the glass flask and the water it contained evaporated. The content had not decomposed as expected, it actually resulted in more product than expected. As further investigations showed, this was based on the fact that the reaction takes place preferentially at temperatures> 100 ° C.
Variant according to Cram
A more modern variant according to Donald J. Cram uses dimethyl sulfoxide as the solvent and potassium tert -butanolate as the base. Since potassium tert -butanolate is more basic than potassium hydroxide , the reaction can be carried out at room temperature.
Similar reactions
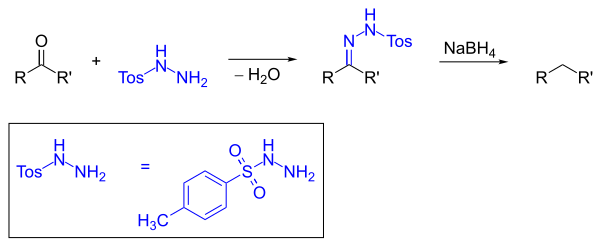
A reaction related to the Wolff-Kishner reaction uses toluenesulfonyl hydrazide as shown below ; the hydrazone formed is then reduced to the alkane with sodium borohydride . The advantage is the avoidance of both strongly acidic and strongly basic reaction conditions, so that reductions in the α-position to chiral centers are possible. However, diaryl ketones cannot be reduced in this way.
The Clemmensen reduction can be seen as complementary to the Wolff-Kishner reaction , in which aldehydes or ketones are reduced by amalgamated zinc to the corresponding methyl or methylene compounds in a hydrochloric acid medium .
literature
- N. Kishner: Journal of the Russian Physico-Chemical Society (J. Russ. Phys. Chem. Soc.), 43, 1911, p. 582.
- L. Wolff: Method for replacing the oxygen atom of the ketones and aldehydes with hydrogen. In: Liebig's annals of chemistry . 394, 1912, pp. 86-108.
- Reinhard Brückner : reaction mechanisms. 3. Edition. Spektrum Akademischer Verlag, Munich 2004, ISBN 3-8274-1579-9 , p. 793.
Individual evidence
- ^ Louis F. Fieser, Mary Fieser: Organic chemistry. 2., verb. Edition. Wiley-VCH, Weinheim, 1989, ISBN 3-527-25075-1 .