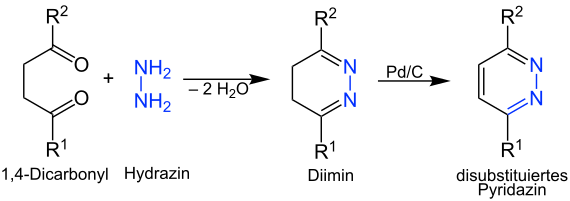Diazines
Diazines are heterocyclic aromatic chemical compounds . They belong to the organic compounds and have a six-membered ring system, which contains two nitrogen atoms as heteroatoms , and thus belong to the azines .
The name diazine can be derived from the systematic Hantzsch-Widman nomenclature .
Three isomeric parent systems can be formulated - pyridazine (1,2-diazine), pyrimidine (1,3-diazine) and pyrazine (1,4-diazine) - whose general empirical formula is C 4 H 4 N 2 .
Occurrence
Diazines can be found in a number of natural products . For example, the nucleobases of DNA and RNA have a purine or pyrimidine structure .
Manufacturing
Pyridazine
A twofold condensation reaction can be used to produce pyridazines . For this purpose, a 1,4-di- carbonyl compound is reacted with hydrazine . Hydrazine condenses on the carbonyl functions in stages, with one particle of water being split off . The formed therefrom diimine can now, for example the palladium - activated carbon - catalyst are dehydrated and be flavored.
Pyrimidines
Pyrimidines can be obtained from the acid-catalyzed reaction of 1,3-dicarbonyl compounds with amidines . In the first step, the amino function condenses on a ketone. The second step consists of a further condensation with subsequent aromatization , whereby the ring is formed. One particle of water is split off in each of the two condensation steps. Both ketones and aldehydes are used as carbonyl components for this reaction.
Pyrazines
Pyrazines can be synthesized by the reaction of carbonyl compounds that are brominated in the α position . The diimine thus obtained can be aromatized oxidatively .
Another possibility is the oxidation of the corresponding piperazines .
properties
Diazines belong to the group of aromatic nitrogen bases . The two nitrogen atoms reduce the electron density in the ring system so much that electrophilic aromatic substitutions often only take place poorly or not at all. The willingness for electrophilic substitution can be increased by introducing electron-donating substituents.
See also
Individual evidence
- ↑ D. Hellwinkel: The systematic nomenclature of organic chemistry , 4th edition, Springer Verlag, Berlin, 1998, ISBN 3-540-63221-2 .
- ↑ Entry on Diazine. In: Römpp Online . Georg Thieme Verlag, accessed on December 28, 2014.
- ^ S. Brooker, RJ Kelly: Synthesis and structure of dilead (II) and dimanganese (II) complexes of macrocycles derived from 3,6-diformylpyridazine , in: J. Chem. Soc. Dalton Trans. 1996 , 10 , 2117-2122; doi : 10.1039 / DT9960002117 .
- ^ HP Latscha, U. Kazmaier, HA Klein: Organische Chemie: Chemie-Basiswissen II , pp. 322–323, 6th edition, Springer Verlag Berlin, 2008, ISBN 3-540-77106-9 .
- ↑ DT Davies: Basic Texts Chemistry: Aromatic Heterocyclen. VCH-Wiley, 1995, ISBN 3-527-29289-6 , pp. 73-77.
- ↑ C. Neuberg: Reduction of amino acids to amino aldehydes , in: Ber. d. German Chem. Ges. 1908 , 41 , pp. 956-963; doi : 10.1002 / cber.190804101185 .
- ↑ C. Stoehr: Ueber Pyrazine und Piperazines , in: J. Prakt. Chem. 1893 , 47 , pp. 439-522; doi : 10.1002 / prac.18930470137 .
- ↑ JA Joules, K. Mills: Heterocyclic Chemistry. 5th edition, Blackwell Publishing, Chichester 2010, ISBN 978-1-405-19365-8 , pp. 250-288.