Pyrimidines
In chemistry, the pyrimidines form a group of organic compounds that belong to the heterocycles (more precisely: heteroaromatic compounds ). They are derived from the parent compound pyrimidine .
If the aromatic π-electron system is retained during the substitution , pyrimidine derivatives in the narrower sense are present, such as, for example, 2,4,6-trimethylpyrimidine or 2,4,6-trichloropyrimidine.
Occurrence and meaning

Substituted pyrimidine rings are partial structures of important natural substances, such as vitamin B 1 ( thiamine ) and the orotic acid found in dairy products , which is also an intermediate stage in pyrimidine de novo synthesis . From the barbituric a number of important pharmaceuticals are derived from the so-called barbiturates , which are substituted on the fifth carbon atom and as a sleep aid used. Pyrimidine derivatives also play a role as diuretics , antibiotics , antimetabolic agents and in antiviral therapy.
However, the building blocks of the nucleic acids of every cell must be considered as the most important derivatives of pyrimidine .
biochemistry
Pyrimidine bases
50% of the so-called "bases" of every DNA molecule contain the pyrimidine framework. During hydrolytic degradation of the polymeric DNA, uracil , thymine (= methyluracil) and cytosine were isolated.
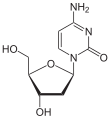
If cytosine and uracil are linked in position 1 to the first carbon atom (C-1) of the ribose , the nucleosides cytidine and uridine result . Esterification of the ribose with phosphate creates the nucleotides CMP and UMP, which are the building blocks of RNA . If the nucleotide contains cytosine or thymine and deoxyribose , it is the building blocks of DNA , deoxy-cytidine (dCMP) and deoxy- thymidine monophosphate (dTMP).
| Nucleobase | Nucleoside | Deoxynucleoside |
|---|---|---|

|

|
|
| Cytosine | Cytidine , C. | Deoxycytidine , dC |

|

|

|
| Thymine | Ribothymidine T (= 5-methyluridine) | Deoxythymidine , dT |

|

|

|
| Uracil | Uridine , U | Deoxyuridine , dU |
In biology and biochemistry, the heterocycles uracil and thymine are referred to as "bases" (" nucleobases ") . However, this does not correspond to the usual definition of a base in chemistry. So has Uracil a pK s value of 9.45 and thus is a weak acid. Cytosine, on the other hand, contains an amino group , which gives the molecule a base character. The biological function in nucleic acids is based less on basicity than on the ability of the "pyrimidine bases" to form hydrogen bonds (H-bonds), and they can act both as H- bond acceptors and as H-bond donors .
The compounds themselves are also strong hydrogen-bonding agents and therefore have very high melting points: uracil 335 ° C, thymine 321 ° C, cytosine 320-325 ° C. They form hydrates (water of crystallization molecules are apparently fixed by hydrogen bonds).
biosynthesis
The heterocyclic ring is built up independently of the ribose and is only linked to the sugar after completion. Orotic acid appears as an intermediate product of this synthesis chain , the end product is uridine monophosphate (UMP), which is converted into CMP , dUMP, dCMP and dTMP in further steps .
The production of pyrimidines in the body takes place in the cytosol and begins with the formation of carbamoyl phosphate from glutamine , 2 ATP and HCO 3 - to carbamoyl phosphate + 2 ADP + Pi + glutamate . The enzyme aspartate carbamyl transferase (ATCase) then catalyzes the formation of N- carbamoyl aspartate from carbamoyl phosphate and aspartate . The ring is then formed from this: N- carbamoyl aspartate cyclizes with elimination of water to form dihydroorotate, which becomes orotate through further elimination of water . The pyrimidine nucleotides are then synthesized from the free pyrimidine orotate.
In mushrooms such as baker's yeast or Candida albicans , pyrimidine can be produced in a completely different way, in that a histidine residue and pyridoxal phosphate ligate to form the pyrimidine ring in the suicide enzyme pyrimidine synthase .
Degradation and modification of the bases
The "bases" are first split off from the nucleosides and nucleotides. These are broken down into β-alanine or 3-aminoisobutyrate .
By nitrous acid (HNO 2 , the amino group of the cytosine is in a) hydroxyl group converted. This creates uracil from cytosine. Acting nitrous acid to the DNA (as a mutagen , performs this change to that in the multiplication of DNA () a reduplication ) to incorrect base pairing occurs and thus to a change in base sequence to changes in proteins and hence to a modified phenotype may result .
Use in medicine
In the past, 6-sulfa-2,4-diemethyl-pyrimidine (sulfadimetin, trade names Aristamid , Elkosin ) was used as a sulfonamide to treat infections. Further standard sulfonamides belonging to the pyrimidines are or were sulfadiazine , sulfamerazine and sulfamethazine . If the synthesis of thymidine is disrupted, this also has the effect of disrupting DNA synthesis. An effective option is to inhibit thymidylate synthase with 5-fluorouracil , which differs from thymine in that it has a fluorine atom in the place of the methyl group . This means that an effective cancer drug is available. However, the disruption of cell division also affects other rapidly growing cells in the hair follicles and bone marrow , which explains the serious side effects of chemotherapy .
5-Fluorocytosine , an antifungal agent, is also based on a pyrimidine structure .
Nomenclature and Tautomerism
Uracil, barbituric acid, etc. are actually only formally pyrimidines; they are descendants of partially hydrogenated pyrimidines. In terms of structure, they can be viewed better as ring-shaped urea derivatives (or lactams or imides ). The structural element of the enamines can also be seen in the formulas of these heterocycles. The phenomenon of tautomerism occurs in these molecules . For example, uracil can be seen formally as 2,4-dihydroxypyrimidine; however, the X-ray crystal structure analysis shows that in the solid state it does not contain any hydroxyl but two oxo groups. The same applies to barbituric acid (formally 2,4,6-trihydroxypyrimidine), cytosine, thymine and orotic acid (figure). The molecules contain the basic pattern N = C – X – H (with X = O, S or NH). The oxo-tautomers have the carboxamide (X = O), thioamide (X = S) or amidine function (X = NH).
The rational nomenclature of these compounds is not very practical, they would be pyrimidinones; Uracil and thymine are pyrimidinediones, cytosine is an aminopyrimidinone. X-ray crystal structure analysis of the three compounds proved the structures for the solid state. Spectroscopic investigations have shown that these structures are predominantly present even in solutions.
Conceivable and previously frequently discussed tautomeric forms with “real”, i.e. H. heteroaromatic pyrimidine framework, are present in solution at best in very small proportions. Nevertheless, the pyrimidinones are often referred to as hydroxypyrimidines, i. H. 2,4-dihydroxypyrimidine (uracil), 2,4-dihydroxy-5-methylpyrimidine (thymine) and 4-amino-2-hydroxypyrimidine (cytosine); Better would be, according to the principles of the suffix nomenclature, as usual in English, the names pyrimidine-2,4-diol etc.
Syntheses of pyrimidines
Of the numerous syntheses, only one important synthetic principle is considered here: the reaction of 1,3-dicarbonyl compounds (or analogous 1,3-bifunctional derivatives) with an amidine or with urea . Here, the six-membered heterocycle is built up from a C 3 component and an NCN component. For example, the condensation of pentane-1,3-dione (“acetylacetone”) with acetamidine gives 2,4,6-trimethylpyrimidine.
The pyrimidinones can also be synthesized from urea as cyclic urea derivatives . Note that the compounds also contain the structural element of enamines , better enamides. Therefore, 1,3-dicarbonyl compounds are used as reactants. For example, uracil can be obtained by condensing urea with 3-oxopropanoic acid ("formyl acetic acid", C 3 H 4 O 3 ). The synthesis is also considered an early proof of structure. In this case, however, the C 3 component cannot be stored and was therefore replaced by malic acid . This is decarbonylated in concentrated sulfuric acid with elimination of water , so it loses carbon monoxide . The 3-oxopropanoic acid formed in situ condenses with the urea in the sulfuric acid solution with two-fold elimination of water.
Fused pyrimidine derivatives
The substance classes of flavins , pteridines and purines are formally derived from the pyrimidines . In addition to the pyrimidine ring, these contain a second heterocyclic ring with a common bond.
literature
- Hans Beyer and Wolfgang Walter : Textbook of Organic Chemistry , 21st edition, S. Hirzel Verlag, Stuttgart 1988, ISBN 3-7776-0438-0 .
Individual evidence
- ↑ Löffler, Petrides, Heinrich: Biochemistry and Pathobiochemistry. 8th edition. Springer, Heidelberg 2007, ISBN 978-3-540-32680-9 .
- ↑ Rung-Yi Lai, Siyu Huang and a .: Thiamin Pyrimidine Biosynthesis in Candida albicans: A Remarkable Reaction between Histidine and Pyridoxal Phosphate. In: Journal of the American Chemical Society. 134, 2012, pp. 9157-9159, doi : 10.1021 / ja302474a .
- ↑ Karl Wurm, AM Walter: Infectious Diseases. In: Ludwig Heilmeyer (ed.): Textbook of internal medicine. Springer-Verlag, Berlin / Göttingen / Heidelberg 1955; 2nd edition, ibid. 1961, pp. 9-223, here: pp. 43-46.
- ^ A. Bowman: Notes: Some Pyrimidine Derivatives , in: J. Chem. Soc. , 1937 , pp. 494-495; doi : 10.1039 / JR9370000494 .








