Sulfonamides

Sulfonamides (chemically more precisely sulfanilamides ) are a group of synthetic chemical compounds with an antimicrobial effect. Some representatives are therefore used as antibiotics . Their effect is based on the fact that they prevent the bacteria from producing folic acid , which is necessary for the production of nucleotides , the basic building blocks of genetic material . This does not kill bacteria directly, but prevents them from multiplying because they cannot copy their genetic information. Structurally, the sulfonamides are descendants of 4-aminobenzenesulfonic acid amide ; they belong to the large group of sulfonic acid amides , which is characterized by the group —SO 2 NHR–. The term chemotherapeutic agent , which is also still used for such active substances, comes from the beginnings of the chemical-synthetic production of antimicrobial substances .
Substances structurally related to the sulfonamides are the sulfonylureas , which are used as oral antidiabetic agents, and the thiazide diuretics .
The effect of the sulfonamides as antimicrobial agents for the treatment of infectious diseases is based on the fact that they act as antimetabolites of p -aminobenzoic acid (PABA). They competitively inhibit the dihydropteroate synthase of the metabolic pathway of folic acid synthesis in bacteria, which catalyzes the formation of dihydropteroic acid. Eukaryotic (and therefore human) cells are not affected by this, as they do not produce folic acid. The effectiveness is also based on the structural similarity of the sulfonamides with carboxamides , the carbonyl group of which is replaced by a sulfonyl group ; so they are analogues of amides . Typical representatives are sulfamethoxazole , the longer-acting sulfadoxine , the sulfacarbamide with a shorter duration of action or sulfasalazine , which is not absorbed in the intestine . Sulphanilic acid itself has no significant effect against bacteria, as it can hardly penetrate the membrane of the microorganisms due to its high polarity.
Manufacturing
Different syntheses have been developed for the production of the sulfonamides. The availability of the starting chemicals and a simple and problem-free course of the intermediate steps are decisive for the type of synthesis chosen. Two possible syntheses for sulfapyridine are shown below as an example for the preparation of sulfonamides .
- Condensation of p -acetamino-benzene-sulfochloride with an amine and subsequent hydrolysis of the acetyl group :
or as a second option:
- Condensation of p -nitrobenzenesulfonic acid chloride with an amine and subsequent reduction of the nitro group :
history
Development of synthetic antibiotics
With the scientific research and synthesis of organic compounds in the 19th century, the search for compounds with antibacterial properties began. Paul Ehrlich was one of the first chemists to systematically examine chemical compounds for their effect on bacteria. Ehrlich's main focus was on azo dyes and similar compounds. After investigations with the azo dye trypan red and the arsenic-containing compound atoxyl , he suspected that structures with azo groups (-N = N-) as well as arsenic compounds (-As = As-) are particularly suitable. For this reason, many new compounds containing arsenic were synthesized and tested in his laboratory.
For Salvarsan , which was developed in his laboratory in 1909 , Ehrlich and the Japanese bacteriologist Sahachiro Hata were able to prove the antibacterial effect on spirochetes and trypanosomes until 1910 . With Salvarsan , a causal treatment and cure of syphilis was possible for the first time with justifiable toxic side effects. The Salvarsan developed in Germany was patented and known as one of the first fully synthetic drugs. The patent was confiscated by the United States during World War I and the compound continued to be used under the name of arsphenamine .
As early as 1908, Paul Gelmo had developed sulfanilamide, the first representative from the sulfonamide group. Heinrich Hörlein , who had already used sulfonamides with azo structure (-N = N-) as textile dyes, was the driving force behind a research program by IG Farben for the systematic development of antibacterial compounds from the group of dyes from coal tar chemistry based on the model from Ehrlichs Salvarsan.
In 1932, the chemists Fritz Mietzsch and Josef Klarer synthesized a sulfonamide within this program, which later became known under the brand name Prontosil . Its antibacterial effect was discovered shortly afterwards (in December 1932) by the physician Gerhard Domagk , who was researching the medical effects of azo dyes for IG Farben at Bayer's main plant in Wuppertal (Elberfeld) and who worked closely with his laboratory for experimental pathology in Elberfeld the chemists worked together. Outside the living organism ( in vitro ), the initially synthesized KL 695 proved to be largely ineffective against streptococci, but it did so in vivo in mice (KL 695 later did not go into clinical tests). The same applied to the variant Kl 730 , which was synthesized and tested shortly afterwards , later called Streptozon and finally Prontosil . In particular, the proof of the effect in the animal model was due to Domagk, who also successfully treated his four-year-old daughter , who had sepsis, with the new drug in December 1933. The development was initially kept as secret as possible, but a patent application was filed as late as Christmas 1932 (published in January 1935).
It was not until February 1935 that Domagk published his studies on the medical effectiveness of Prontosil and thus for the first time a description of the chemotherapeutic effects of sulfonamides. In 1939 Domagk was awarded the Nobel Prize for his work , which he was not allowed to accept due to the laws of the time of National Socialism. One reason for the delay in development was the opposition of the National Socialists, who came to power in 1933, to animal experiments.
The mechanism of action of Prontosil was clarified in 1935 by Jacques Tréfouël , Thérèse Tréfouël, Federico Nitti and Daniel Bovet in the laboratory of Ernest Fourneau : Prontosil is only metabolized in the organism to the pharmacologically active form, sulfanilamide, which explains its ineffectiveness in vitro . There was later a dispute about whether the Bayer scientists had also recognized the importance of the sulfanilamide component for the antibacterial effect. Bovet took the view that they only came up with it through the work of the French scientists at the Pasteur Institute, because they believed there was a connection between dye properties and antibacterial properties. Testing in bacteriological laboratories did not begin until 1936, clinical tests at IG Farben did not begin until March 1936 and later in 1936 it came onto the market as the Prontosil album ( album for colorless). The British bacteriologist Ronald Hare, on the other hand, took the view in 1970 that this was already known to Bayer scientists, but that they were looking for a component that was patentable.
The sulfonamides were the first broad-spectrum antibiotics that were successfully used in medicine. It was not until later, in the Second World War from 1940, that Florey and Dunn also introduced penicillin into medical therapy.
Over 1000 sulfonamidic compounds had been synthesized by the late 1930s. However, only a few of them are pharmacologically effective. All the nucleus-substituted derivatives of sulfanilamide tested so far are completely ineffective. On the other hand, compounds that have the following structure are particularly effective:
contain.
The sulfonamides (the year of introduction in brackets), sulfapyridine (1938), sulfathiazole (1940), sulfaguanidine (1940), sulfadiazine (synonym: sulfapyrimidine, 1941), phthalylsulfathiazole (1942) and mono- and dimethyl derivatives of sulfathiazine were widely used ( 1943).
The first drug with a sulfonamide active ingredient was Prontosil ( sulfamidochrysoidin ). Apart from Prontosil , most sulfonamides are effective both in vitro and in vivo . The antibacterial mechanism of action of sulfonamides as antimetabolites was elucidated in 1940 by Donald D. Woods and Paul Fildes .
In the course of the widespread use of sulfonamides in antimicrobial therapy, further effects were discovered in some representatives, which justified the development of further classes of active ingredients. So the discovery of the diuretic (led in the 1940s diuretic ) effect, particularly the sulfonamide sulfanilamide to the development of new drug class of " thiazide diuretics ". The preparation Haflutan (6-chloro-benzene-1,3-disulfonamide), which stimulates the urine flow, also became known . Because of its blood sugar-lowering effect, the sulfonamide carbutamide came onto the market in 1956 as the first representative of the “ sulfonylureas ” used in antidiabetic therapy .
Penicillin and the various other compounds of this type have now largely replaced the sulfonamides in medical use, as they are safer with lower doses. The compounds sulfamethoxazole , silver sulfadiazine and sulfamerazine are among the few sulfonamides still used today in medicine for humans . In veterinary medicine, on the other hand , especially for the treatment of diseases caused by parasite infestation , combination preparations with sulfonamides are still often used.
Attempts at the time of National Socialism
At the time of National Socialism , medical experiments were carried out on concentration camp prisoners in the Ravensbrück and Dachau concentration camps . The background was that Reinhard Heydrich died of sepsis while he was under the supervision of Himmler's personal doctor Karl Gebhardt . Hitler's personal physician Morell had criticized that Heydrich might have survived if the sulfonamide Ultraseptyl had been used. At Heydrich, however, other sulfonamides were administered, Gebhardt got into trouble and initiated the experiments. Injuries were inflicted on concentration camp inmates and wounds were infected in order to achieve sepsis and to test the effectiveness of various sulfonamides. Gebhardt was sentenced to death , among other things, for these attempts at the Nuremberg medical trial .
Table sulfonamides
The following table gives an overview of the first simpler compounds and the sulfonamides that are still used today. Mafenide is also included in the sulfonamides, although it does not have a sulfanilamide structure. In addition, other representatives of this class of active ingredients not listed here were and are also used in medicine. Mixtures of two or more sulfonamides or other active substances are predominantly used as antibiotics. Almost all of the individual compounds have many synonyms - in some cases well over 20 - in use for the names.
| structure | INN name |
CAS no. PubChem |
Molecular formula | IUPAC name | use |
|---|---|---|---|---|---|
|
|
Sulfanilamide | 63-74-1 5333 |
C 6 H 8 N 2 O 2 S | 4-aminobenzenesulfonamide | Basic building block of the sulfonamides |
|
|
Sulfathiourea (synonym: sulfathiocarbamide) | 515-49-1 3000579 |
C 7 H 9 N 3 O 2 S 2 | (4-aminophenyl) sulfonylthiourea | hardly used anymore ( badional ) |
|
|
Sulfacarbamide | 547-44-4 11033 |
C 7 H 9 N 3 O 3 S 2 | (4-aminophenyl) sulfonylurea | in human medicine ( Euvernil ) |
|
|
Mafenid | 138-39-6 3998 |
C 7 H 10 N 2 O 2 S | 4- (aminomethyl) benzenesulfonamide | in veterinary and human medicine (burns) |
|
|
Sulfaguanidine | 57-67-0 5324 |
C 7 H 10 N 4 O 2 S | 4-amino- N - (diaminomethylene) benzenesulfonamide | in veterinary medicine (rarely used) |

|
Sulfacetamide | 144-80-9 5320 |
C 8 H 10 N 2 O 3 S | N - [(4-aminophenyl) sulfonyl] acetamide | in human medicine (eyes) |
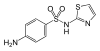
|
Sulfathiazole | 72-14-0 5340 |
C 9 H 9 N 3 O 2 S 2 | 4-amino- N - (1,3-thiazol-2-yl) benzenesulfonamide | in veterinary medicine |
|
|
Sulfamethizole | 144-82-1 5328 |
C 9 H 10 N 4 O 2 S 2 | 4-Amino- N - (5-methyl-1,3,4-thiadiazol-2-yl) benzenesulfonamide | in veterinary medicine |

|
Sulfametrol | 32909-92-5 64939 |
C 9 H 10 N 4 O 3 S 2 | 4-amino- N - (4-methoxy-1,2,5-thiadiazol-3-yl) benzenesulfonamide | in human medicine |

|
Sulfamethylthiazole | 515-59-3 5328 |
C 10 H 11 N 3 O 2 S 2 | 4-Amino- N - (4-methyl-1,3-thiazol-2-yl) benzenesulfonamide | in human medicine |
|
|
Sulfachloropyridazine | 80-32-0 6634 |
C 10 H 9 ClN 4 O 2 S | 4-amino- N - (6-chloropyridazin-3-yl) benzenesulfonamide | in veterinary medicine |
|
|
Sulfachlorpyrazine | 1672-91-9 164867 |
C 10 H 9 ClN 4 O 2 S | 4-amino- N - (5-chloropyrazin-2-yl) benzenesulfonamide | in veterinary medicine for poultry |

|
Sulfadiazine | 68-35-9 5215 |
C 10 H 10 N 4 O 2 S | 4-amino- N -pyrimidin-2-ylbenzenesulfonamide | in human (skin) and veterinary medicine |
|
|
Sulfamethoxazole | 723-46-6 5329 |
C 10 H 11 N 3 O 3 S | 4-Amino- N - (5-methyl-1,2-oxazol-3-yl) benzenesulfonamide | in human and veterinary medicine |

|
Sulfapyridine | 144-83-2 5336 |
C 11 H 11 N 3 O 2 S | 4-amino- N -pyridin-2-yl-benzenesulfonamide | in human medicine (skin) |
|
|
Sulfamerazine | 127-79-7 5325 |
C 11 H 12 N 4 O 2 S | 4-amino- N - (4-methylpyrimidin-2-yl) benzenesulfonamide | in veterinary medicine |
|
|
Sulfaperine | 599-88-2 68933 |
C 11 H 12 N 4 O 2 S | 4-Amino- N - (5-methylpyrimidin-2-yl) benzenesulfonamide | no longer applied |
|
|
Sulfamethoxypyridazine | 80-35-3 5330 |
C 11 H 12 N 4 O 3 S | 4-amino- N - (6-methoxypyridazin-3-yl) benzenesulfonamide | in veterinary medicine |
|
|
Sulfamethoxydiazine | 651-06-9 5326 |
C 11 H 12 N 4 O 3 S | 4-amino- N - (5-methoxypyrimidin-2-yl) benzenesulfonamide | in human medicine |
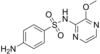
|
Sulfals | 152-47-6 9047 |
C 11 H 12 N 4 O 3 S | 4-amino- N - (3-methoxypyrazin-2-yl) benzenesulfonamide | in veterinary and human medicine |
|
|
Sulfamoxol | 729-99-7 12894 |
C 11 H 13 N 3 O 3 S | 4-Amino- N - (4,5-dimethyl-1,3-oxazol-2-yl) benzenesulfonamide | in human medicine |
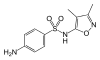
|
Sulfafurazole (Synonym: Sulfisoxazole ) | 127-69-5 5344 |
C 11 H 13 N 3 O 3 S | 4-Amino- N - (3,4-dimethyl-1,2-oxazol-5-yl) benzenesulfonamide | in human medicine |
|
|
Sulfadicramide | 115-68-4 8281 |
C 11 H 14 N 2 O 3 S | N - (4-aminophenyl) sulfonyl-3-methylbut-2-enamide | in human medicine (hardly used anymore) |
|
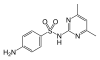
Sulfadimidine | 57-68-1 5327 |
C 12 H 14 N 4 O 2 S | 4-amino- N - (4,6-dimethylpyrimidin-2-yl) benzenesulfonamide | in veterinary medicine |
|
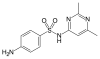
Sulfisomidine | 515-64-0 5343 |
C 12 H 14 N 4 O 2 S | 4-Amino- N - (2,6-dimethylpyrimidin-4-yl) benzenesulfonamide | in human medicine |
|
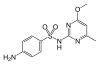
Sulfametomidine | 3772-76-7 19596 |
C 12 H 14 N 4 O 3 S | 4-Amino- N - (6-methoxy-2-methylpyrimidin-4-yl) benzenesulfonamide | in human medicine |

|
Sulfadimethoxine | 122-11-2 5323 |
C 12 H 14 N 4 O 4 S | 4-amino- N - (2,6-dimethoxypyrimidin-4-yl) benzenesulfonamide | still in veterinary medicine. former trade name Madribon |
|
|
Sulfadoxine | 2447-57-6 17134 |
C 12 H 14 N 4 O 4 S | 4-amino- N - (5,6-dimethoxypyrimidin-4-yl) benzenesulfonamide | in veterinary medicine |

|
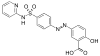
Sulfaphenazole | 526-08-9 5335 |
C 15 H 14 N 4 O 2 S | 4- Amino- N - (1-phenyl-1 H -pyrazol-5-yl) benzenesulfonamide | in veterinary medicine |
|
Sulfasalazine | 599-79-1 5359476 |
C 18 H 14 N 4 O 5 S | 2-Hydroxy-5 - [( E ) -4- (pyridin-2-ylsulfamoyl) phenyldiazenyl] benzoic acid | in human and veterinary medicine |
properties
Sulphonamides have a bacteriostatic effect . If they are used individually, resistances develop quickly . They are therefore preferably combined with dihydrofolic acid reductase inhibitors such as trimethoprim ( cotrimoxazole see below). Sulphonamides are relatively hydrophilic, can be given orally (e.g. as a tablet ) and are mainly excreted via the kidneys with different half-lives . They share the process of secretion in the renal tubules with some other acids , which then hinder each other in their excretion: uric acid , uricosurics and uricostatics , acetylsalicylic acid , thiazide diuretics , penicillin and macrolides . The elimination route also means that they accumulate in the urinary system, which is also their main area of application.
application
Sulphonamides act against intestinal bacteria , for example Escherichia coli , Pseudomonas , Salmonella , Shigella . They are also effective against Staphylococcus , Streptococcus , Neisseria , Pneumocystis jirovecii , Toxoplasma gondii , Neospora caninum and Plasmodia . They are mainly used as co- trimoxazole against uncomplicated urinary tract infections and against pneumonia caused by Pneumocystis jirovecii (formerly: P. carinii ), here as the only drug. Furthermore, they are used in various combinations against toxoplasmosis or malaria in need of therapy. However, sulfonamides are ineffective against Bacteroides , Enterococcus , mycoplasma , fungi , Pseudomonas aeruginosa , most protozoa , rickettsiae and viruses .
In veterinary medicine , sulfonamides are common antibiotics that are used for respiratory, gastrointestinal and urinary tract diseases. Furthermore, they are often the means of choice for the treatment of rodents , since these generally have a broad intolerance to antibiotics (especially penicillin ). In the poultry sector, sulfonamides have long been the means of combating coccidia .
Sulfonamides are mostly used in combination with diaminopyrimidines . A fixed combination of the substances sulfamethoxazole plus trimethoprim in a ratio of 5 to 1 is called cotrimoxazole . The combination is intended to prevent the development of antibiotic resistance , and the potency of antibiotics that block the same metabolic pathway at different points increases. In Germany, only sulfadiazine is used for monotherapy for the treatment of acute and recurrent toxoplasmosis .
Legal provisions
The group of sulfonamides is summarized in Table 1 of Regulation (EU) No. 37/2010 on pharmacologically active substances and their classification with regard to the maximum residue levels in foods of animal origin and as such may only occur in certain maximum amounts in various animal foods. The following table summarizes the legal requirements.
| Pharmacologically active substance (s) | Marker residue | Animal species | Maximum residue level (s) | Target tissue | Other regulations (according to Article 14 (7) of Regulation (EC) No. 470/2009 ) | Therapeutic classification |
|---|---|---|---|---|---|---|
| Sulfonamides (all substances of the sulfonamide group) | Parent compound | All types used for food production | 100 µg / kg | muscle
fat liver Kidneys |
The residues of all substances of the sulfonamide group must not exceed a total of 100 µg / kg.
For fish, the MRL relates to "muscle and skin in natural proportions". The maximum residue limits for fat, liver and kidneys do not apply to fish. Not for use in animals whose eggs are intended for human consumption. |
Agents against infections / chemotherapy drugs |
| Cattle, sheep, goats | 100 µg / kg | milk |
Interactions
When local anesthetics from the group of para- aminobenzoic acid esters (for example procaine or tetracaine ) are administered at the same time, antagonism occurs , since these cancel out the effect of sulfonamides. Furthermore, there are undesirable interactions with methenamine and in some cases with phenylbutazone .
Side effects
Sulphonamides are not approved for pregnancy , especially around the due date, as they can cause dangerous hyperbilirubinemia in the newborn . On the other hand, there can be allergies , especially on the skin - here sulfonamides can also trigger strong phototoxicity . Changes in the blood count or hepatic cholestasis , as with sulfonylureas, are rare . In patients with hereditary methaemoglobinemia "medication with sulfonamides leads to severe hemolytic crises."
In addition, the formation of aqueous humor is inhibited, as sulfonamides inhibit the effect of carbonic anhydrase . This leads to an effective reduction in eye pressure . Eye drops with modified sulfonamides are therefore used today as the first choice in the treatment of glaucoma .
Remarks
- ^ SY Hwang, DA Berges, JJ Taggart, C. Gilvarg: Portage transport of sulfanilamide and sulfanilic acid . In: Journal of Medicinal Chemistry . tape 32 , no. 3 , March 1989, p. 694-698 , doi : 10.1021 / jm00123a034 , PMID 2645404 .
- ↑ a b c d e f L. F. Fieser, M. Fieser: In: Textbook of organic chemistry. 3. Edition. Verlag Chemie, Weinheim ad Bergstrasse 1957, p. 1191.
- ↑ a b L. F. Fieser, M. Fieser In: Textbook of organic chemistry. 3. Edition. Verlag Chemie, Weinheim ad Bergstrasse 1957, p. 1193.
- ↑ LF Fieser, M. Fieser In: Textbook of organic chemistry. 3. Edition. Verlag Chemie, Weinheim ad Bergstrasse 1957, p. 1182.
- ↑ Brockhaus of the natural sciences and technology. 4th edition. Brockhaus, Wiesbaden 1958, p. 480.
- ↑ LF Fieser, M. Fieser: In: Textbook of organic chemistry. 3. Edition. Verlag Chemie, Weinheim ad Bergstrasse 1957, p. 1183.
- ↑ Brockhaus of the natural sciences and technology. 4th edition. Brockhaus, Wiesbaden 1958, p. 553.
- ↑ Wolf-Dieter Müller-Jahncke : Sulfonamides. In: Werner E. Gerabek , Bernhard D. Haage, Gundolf Keil , Wolfgang Wegner (eds.): Enzyklopädie Medizingeschichte. De Gruyter, Berlin / New York 2005, ISBN 3-11-015714-4 , p. 1367.
- ↑ The development of Prontosil is presented in John Lesch, The first miracle drugs , Oxford UP 2007, p. 57ff
- ^ W-Dieter Müller-Jahncke: Sulfonamides. 2005, p. 1367.
- ^ Lesch, The first miracle drugs, p. 82
-
^ Domagk contribution to chemotherapy of bacterial infections. In: German. Med. Weekly. Volume 61, February 15, 1935, pp. 250-253;
Domagk chemotherapy of bacterial infections. In applied chemistry . Volume 46, 1935, pp. 657-667. - ↑ On the history of sulfonamides, see also John Lesch The miracle drugs. Oxford University Press 2006.
- ↑ Lesch, The miracle drugs, Chapter 4, Into the maelstrom, pp. 71ff
- ^ J. and Th. Tréfouël, F. Nitti, D. Bovet, “Activité du p.aminophénylsulfamide sur l'infection streptococcique expérimentale de la souris et du lapin”, CR Soc. Biol. 120, November 23, 1935, p. 756.
- ^ Lesch, The making of the first miracle drugs, p. 84
- ↑ Lesch, The first miracle drugs, pp. 83f
- ↑ Brockhaus of the natural sciences and technology. 4th edition. Brockhaus, Wiesbaden 1958, p. 411.
- ↑ LF Fieser, M. Fieser: In: Textbook of organic chemistry. 3. Edition. Verlag Chemie, Weinheim ad Bergstrasse 1957, p. 1196. Not Dunn but Chain is listed as the second researcher.
- ↑ LF Fieser, M. Fieser: Textbook of organic chemistry. 3. Edition. Verlag Chemie, Weinheim ad Bergstrasse 1957, p. 1194.
- ↑ H. Auterhoff : Textbook of Pharmaceutical Chemistry. WVG 1978, 472 ff.
- ^ Herder Lexicon Chemistry. Licensed edition for Bertelsmann Club, Gütersloh, book no. 03838 0, p. 232.
- ↑ Entry on sulfonamide antibiotics in Flexikon , a Wiki of the DocCheck company , accessed on November 25, 2015 (point 3)
- ^ Zámečník: That was Dachau. Foundation Comité International de Dachau, Luxembourg 2002, p. 285 ff.
- ↑ all information, unless otherwise stated, from the CliniPharm database
- ↑ PubChem: Sulfathiourea .
- ^ Frank A. Plumer et al .; In: N Engl. J. Med .; 1983, 309, pp. 67-71.
- ↑ Entry on sulfamerazine at Vetpharm, accessed on June 23, 2012.
- ↑ The Clinical Performance of Sulfamoxole, A New Sulfonamide Analysis of 1240 Case Reports ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. .
- ↑ www.vetpharm.uzh.ch .
- ↑ MedlinePlus Drug Information: Erythromycin and Sulfisoxazole .
- ↑ Anderson EA, Knouff EG, Bower AG; In: Calif. Med. 1956 May; 84 (5): 329-30.
- ↑ Junyi Xu & Claudia Gallert & Josef Winter, Appl Microbiol Biotechnol (2007) 74: 493-500.
- ↑ Entry on sulfonamides. In: Römpp Online . Georg Thieme Verlag, accessed on June 25, 2011.
- ↑ Regulation (EU) No. 37/2010 of the Commission of December 22, 2009 on pharmacologically active substances and their classification with regard to the maximum residue levels in food of animal origin. Retrieved February 6, 2019.
- ↑ M. Buchta: Molecular foundations of human genetics . In: M. Buchta, DW Höper, A. Sönnichsen: Das Hammerexamen. Revision course for the 2nd section of the medical examination. 1st edition. Elsevier, Munich 2006, p. 1426.