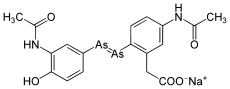
Arsphenamine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| Trimeric form of arsphenamine | |||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Arsphenamine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula |
|
||||||||||||||||||
| Brief description |
Light yellow, somewhat hygroscopic powder that oxidizes in air |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | |||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
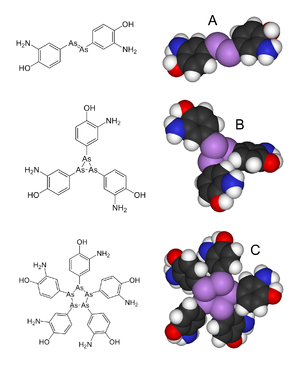
Arsphenamine (also Dioxydiamino-Arsenobenzol or shortly Arsenobenzol ; former brand name: Salvarsan ) is a mixture of several organic arsenic compounds with which the syphilis was first treated by chemotherapy. More precisely, it is a mixture of a trimeric and pentameric cyclic compound (triaminotrihydroxy-arsenobenzene and pentaaminopentahydroxy-arsenobenzene). Paul Ehrlich adopted a dimeric structure in 1912, the (dichloride) 3,3′-diamino-4,4′-dihydroxy-arsenobenzene.
history
The chemist Alfred Bertheim synthesized over 600 arsenic compounds in Paul Ehrlich's laboratory from 1906 onwards . After 606 animal experiments which eventually became preparation 606 (also Ehrlich-Hata preparation and Ehrlich 606 and short 606 ) on 31 August 1909 by Paul Ehrlich and Sahachiro Hata tested positive against the causative agent of syphilis. The first recipients of "Ehrlich-Hata 606" included Konrad Alt (1861–1922), Alfred Blaschko , Wilhelm Wechselmann and some doctors who were personally known to Ehrlich. Konrad Alt, the director of the Uchtspringe sanatorium, began in autumn 1909 with a series of experiments on men with paralysis who were injected with the drug intramuscularly, and Wechselmann's remarks that apart from the painful side effects of the injection there were no complications during the experiments the way for the application of the drug outside of the clinic. The preparation was produced by Hoechst and was officially known as Salvarsan on the market in November 1910 . Ehrlich-Hata 606 was already applied in April 1910 - for example by Heinrich Loeb (* 1862) at the Mannheim City Hospital - in consultation with Paul Ehrlich.
After Loeb had reported 11 relapses in 187 patients in November 1910, however, he switched to the combination of Salvarsan and mercury, and the mercury therapy previously used for syphilis (e.g. with sublimate ) was used again in other clinics , especially Salvarsan in the case of syphilis "Late syphilis" showed hardly any positive results and in 1911 and 1912 the first reports were published on deaths from salvarsan and arsenobenzene , probably caused by other complications (fever- inducing ) pyrogens . Such cases were already referred to as Salvarsantod in 1913/14 . The Viennese dermatologist Ernest Finger proved that the low relapse rates mentioned by the Berlin Speyer-Haus were incorrect. Between 1918 and 1933, doubts were also expressed by various sides about the effectiveness of the drug, which was recognized as having side effects as early as 1910, including liver-damaging and, among other things, also had a negative effect on the central nervous system, and the methods used to determine whether the drug was successful. But even the combination therapy that initially promised success was not always satisfactory.
The name Salvarsan (composed of the Latin words salvare - save, heal, sanus - healthy, unhealthy and a remnant of the word arsenic ) means healing arsenic or " healing arsenic " . In fact, Salvarsan represented a milestone in drug research. For the first time, medicine was provided with a drug with a targeted antimicrobial effect against a dangerous infectious disease . In addition, Salvarsan was used not only against syphilis, but also against framboesia , relapsing fever and other spirochaete infections . Salvarsan was thus one of the first antimicrobial drugs . It was so expensive that it was even worth exporting to the USA in a commercial submarine during the First World War .
The starting point
Paul Ehrlich's research was based on the Atoxyl , which Robert Koch previously reported to be effective against sleeping sickness . From the observation that pentavalent arsenic compounds such as Atoxyl only had a weak effect on germs in the test tube, Ehrlich concluded that the substance is only converted into the actual active substance in the human body . Ehrlich suspected that this must be a trivalent arsenic compound and concentrated his research in this direction. Ehrlich called his development goal the magic ball , using this name to refer to the selective toxicity for certain pathogens.
The search for the magic ball
In the further search carried out specifically by Ehrlich and his employees, modern methods of drug research were used for the first time. Test tube tests and animal experiments were carried out on a large scale in order to be able to examine the largest possible number of compounds. As pathogens served Trypanosoma equinum , the causative agent of mal de calderas , the cross lameness of horses. Salvarsan was finally discovered as the 606th substance tested in the series of tests. This is where the original name for Salvarsan 606 (Dioxy-diamino-arsenobenzene-dihydrochloride) results .
The idea of using the substance against the similar syphilis was probably inspired by a paper by Fritz Schaudinn . After the high potential of the compound had been determined in the following tests and the first clinical tests were successful, the production of the preparation began around a year later at the Hoechst plant.
A basic structure of the Salvarsan was later shown on the 200 DM notes together with its inventor Paul Ehrlich . The substance shown there is hexaphenylcyclohexaarsane , because Ehrlich also received compounds such as (AsAr) n (n = 5, 6, 7; Ar = aryl, i.e. aromatic side group) in the search for active ingredients against syphilis and other infectious diseases.
application
Since Salvarsan oxidizes very quickly to toxic compounds in the air, it was brought onto the market in airtight glass ampoules by the Hoechst paint works and under state control by the Speyer-Haus and Paul Ehrlich. Salvarsan is said to have had a healing effect on some infections with a single injection, but the idea of a single injection was abandoned by doctors who used it as early as 1911, especially since treatment with Salvarsan was still considered an immature procedure in 1915 and especially from 1913, as was its reliability the serological test used by August von Wassermann had been the subject of controversial debate in the context of salvarsan therapy. Among the opponents of the sole use of Salvarsan for syphilis treatment, as it was repeatedly propagated, was among others the Berlin dermatologist and specialist in venereal diseases Heinrich Dreuw (1874-1934), who until May 1914 also worked as a police doctor and in this context in the prostitute examination was active. The debate about the drug also preoccupied politicians. Since it had to be mixed with sodium hydroxide solution before the injection, Salvarsan caused internal burns (damage to veins) when used intravenously or intramuscularly. Therefore better tolerated derivatives of the substance such as 1912, the presented by Victor member Berger (1893 to 1950) supplementary product (were the following years developed Neoarsphenamin called) Neosalvarsan also, preparation 914 called, produced by the action of formaldehyde sulfoxylate on arsphenamine sodium salt of salvarsan , and later the Solu-Salvarsan . A delivered with ranging levels of arsenic and silver Hoechst AG Silbersalvarsan was produced from November 1918th
- Derivatives of salvarsan
It is not yet clear from the sources whether the original Salvarsan was also used against sleeping sickness. Suramin ( Bayer 205 , Germanin ) was developed from these types of medication and is still used today against sleeping sickness.
Salvarsan and its successor products are no longer used today, because from the middle of the 20th century they were largely replaced by modern, safely effective antibiotics such as the sulfonamides traded after 1935 (see also sulfamidochrysoidin : It came on the market in 1932 and was the first antibiotic from the Group of sulfonamides) and penicillin , which came onto the market between 1943 and 1946 , were replaced. The discovery of the drug, however, contributed significantly to the further intensification and improvement of drug research. However, in 1950/51 the Spirotrypan developed in 1939 , which, like Salvarsan, was also used to treat congenital syphilis , was still presented by Hoechst as the “new Salvarsan”. The production of Salvarsan was stopped by Hoechst in 1972.
In 2012, Florian G. Mildenberger summarized his literature research in a closing word: “The Salvarsan never worked, at least not better than mercury or a hydrotherapeutic cure. Symptoms quickly disappeared when the patient survived the side effects, but there was no healing. Wassermann's test procedure produced results of all kinds, just not unique. [...] "
Molecular structure
The monomeric structure of salvarsan was assumed by Ehrlich, but it was later shown by single-crystal structure photographs of unsubstituted arsenobenzene (Ph-As = As-Ph) that this is present as a cyclic trimer . Recent mass spectrometric studies show that salvarsan is present as a cyclic trimer and pentamer.
literature
- Paul Ehrlich (Ed.): Treatises on Salvarsan (Ehrlich-Hata preparation 606 against syphilis). 4 volumes, Munich 1911–1914.
- Salvarsan. In: German Colonial Lexicon. Volume III, 1920, p. 207. HTML
- Robert Bernhardt: Indications and contraindications of the Salvarsan treatment of syphilis. In: Arch. Dermatol. Syphilis. Volume 173, 1936, pp. 291-301.
- Wilhelm Kolle , Karl Zieler (Hrsg.): Handbook of Salvarsan Therapy including the experimental, biological and chemical principles. 2 volumes. Urban & Schwarzenberg, Berlin / Vienna 1924–1925.
- Paul de Kruif: Microbe hunter. 1980, ISBN 3-550-06084-X . (New edition of "Microbe Hunters", 1926/7)
- Fritz Sörgel et al: Which job title does Ehrlich's work justice. In: Chemotherapy Journal. Vol. 13, No. 4, pp. 157-165. 2004. (PDF)
- Nicholas C. Lloyd et al: Salvarsan - The first chemotherapeutic compound. (PDF)
- Florian G. Mildenberger : No salvation through arsenic? The salvarsand debate and its consequences. In: Specialized prose research - Crossing borders. 8/9, 2012/2013, pp. 327-390.
- Lutz Sauerteig: Salvarsan and the "medical police state". Syphilis therapy in the dispute between doctors, the pharmaceutical industry, health administration and naturopathic associations (1910–1927). In: Martin Dinges (Ed.): Movements critical of medicine in the German Reich (approx. 1870 - approx. 1933). Stuttgart 1996, pp. 161-200.
- Lutz Sauerteig: Salvarsan. In: Werner E. Gerabek u. a. (Ed.): Encyclopedia of medical history. De Gruyter, Berlin / New York 2005, ISBN 3-11-015714-4 , p. 1282 f.
- Hans Theodor Schreus : Salvarsan - Review and Outlook. In: Dermatologische Wochenschrift. Volume 138, 1958, pp. 1353-1359.
- Doris Schwarzmann-Schafhauser: Arsenic. In: Werner E. Gerabek u. a. (Ed.): Encyclopedia of medical history. 2005, p. 101.
Web links
- Paul Ehrlich - From Immunology to Salvarsan . In: Pharmaceutical newspaper . 11/2004.
Individual evidence
- ↑ a b c Entry on arsphenamine. In: Römpp Online . Georg Thieme Verlag, accessed on May 8, 2014.
- ↑ Entry on arsenic compounds in the GESTIS substance database of the IFA , accessed on February 1, 2016 (JavaScript required)
- ↑ Not explicitly listed in Regulation (EC) No. 1272/2008 (CLP) , but with the specified labeling it falls under the group entry arsenic compounds, with the exception of those named in this appendix in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA) on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Entry on arsphenamine in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ Florian G. Mildenberger: No salvation through arsenic? The salvarsand debate and its consequences. 2012/13, p. 327.
- ↑ Amanda Yarnell: Salvarsan. Chemical & Engineering News, accessed December 7, 2010.
- ↑ Paul Ehrlich , Alfred Bertheim : About the hydrochloric acid 3.3-diamino-4.4-dioxy-arsenobenzene and its closest relatives. In: Reports of the German Chemical Society. Volume 45, No. 1 1912, pp. 756-766, doi: 10.1002 / cber.191204501110 .
- ↑ Julius Iversen: About the treatment of syphilis with the preparation "606" Ehrlichs. In: Paul Ehrlich (Ed.): Treatises on Salvarsan […]. Volume 1, 1911, pp. 150-154.
- ↑ Stefan Winkle: Cultural history of epidemics . Komet, Düsseldorf / Zurich 1997, ISBN 3-933366-54-2 , p. 599-602 .
- ↑ Konrad Alt: The newest Ehrlich-Hata preparation against syphilis. In: Münchner medical Wochenschrift. Volume 57, 1910, pp. 561-564.
- ↑ Konrad Alt: The newest Ehrlich-Hata preparation against syphilis. In: Munich Medical Weekly. Volume 57, 1910, pp. 561-564.
- ↑ Wilhelm Wechselmann: About local and general hypersensitivity when using Dioxydiamidoarsenobenzol (Ehrlich 606). In: Berlin clinical weekly. Volume 47, 1910, pp. 2133-2137.
- ↑ Kurt v. Stokar: Syphilis treatment with Salvarsan (Ehrlich Hata 606) together with a systematic summary of the literature published so far. Munich 1911.
- ↑ E. Tomaszewski: Summary overview of the Salvarsan treatment of syphilis. Berlin / Vienna 1911.
- ↑ Rockefeller Archive Center: Paul Ehrlich Collection, 650Eh89, Box 51, Folder 6, September 1, 1911, letter, handwriting, Mannheim, Heinrich Loeb to Paul Ehrlich.
- ^ Alfred von Decastello: case of highly febrile tertiary syphilis. Scientific Medical Society in Innsbruck, meeting on June 30, 1910. In: Wiener Klinische Wochenschrift. Volume 23, 1910, p. 1159 f.
- ↑ Carl Bruck : About the successes with the one-time combined Salvarsan sublimate treatment of syphilis according to Linser. In: Münchner medical Wochenschrift. Volume 67, 1920, pp. 423-424.
- ↑ Walther Schönfeld : About the one-time combined intravenous mercury salvarsan treatment of syphilis with special consideration of Novasurol silver salvarsan mixtures. In: Munich medical weekly. Volume 68, 1921, pp. 197-199.
- ↑ Karl Zieler : Development and results of modern arsenic therapy for syphilis. In: Paul Ehrlich (ed.): Treatise on Salvarsan (Ehrlich-Hata preparation 606 against syphilis). Collected and published with a foreword and concluding remarks. Volume 1, Munich 1911, pp. 3–16, here: p. 10.
- ↑ Axel Jorgensen: A case of fatal arsenic poisoning in the treatment of brain syphilis (dementia paretica) with Ehrlich-Hata 606. In: Medical Clinic. Volume 7, 1911, pp. 372-374.
- ^ Karl Martius: About deaths after salvarsan injections in cardiovascular diseases. In: Paul Ehrlich (Ed.): Treatises on Salvarsan. Collected and published with a foreword and concluding remarks. Volume 2, Munich 1912, pp. 410-423.
- ↑ See also Ludwig Arzt, Wilhelm Kerl : To the criticism of the views on the origin of the Salvarsan fever. In: Wiener Klinische Wochenschrift. Volume 24, 1911, pp. 1663-1665.
- ↑ Paul Ehrlich: About the preparation of the salvarsan. 30 letters from Paul Ehrlich to Hoechst. A contribution to modern galenics. Hoechst: Farbwerke formerly Meister Lucius & Brüning, Frankfurt am Main 1966, p. 10 and 66.
- ^ I. Gyula Fazekas, A. Dosa: Histological changes in arsenobenzene deaths and their evaluation. In: Arch. Dermatol. Syph. Volume 197, 1957, pp. 436-448.
- ^ Fritz Lube: About deaths after Salvarsan. In: German Medical Science. Volume 41, 1916, p. 1462 f.
- ^ Leo von Zumbusch : Deaths after Salvarsan injections. In: Munich medical weekly. Volume 63, 1916, pp. 750-753.
- ↑ Theo v. Marschalko, D. Veszpremi: Histological and experimental studies on the Salvarsantod. In: Arch. Dermatol. Syph. Volume 114, 1913, pp. 589-610.
- ↑ Carl Schindler: The Salvarsantod. Its cause and its prevention. Intravenous or intramuscular injection of salvarsan. Berlin 1914.
- ↑ Arthur Schmitt: The Salvarsan deaths and their causes with consideration of the Salvarsan damage. In: Munich medical weekly. Volume 61, 1914, pp. 1337-1340.
- ↑ I [stván] Gyula Fazekas, A. Dosa: Contributions to the mechanism of Salvarsantodes. In: Arch. Dermatol. Syph. Volume 198, 1954, pp. 89-102.
- ↑ Max Lissauer: On the question of the Salvarsan death. In: German Medical Weekly . Volume 43, 1917, p. 1471 f.
- ^ Otto Loeb: Salvarsantod and flu. Negotiations of the German dermatological society, twelfth congress held in Hamburg 17. – 21. May 1921. In: Arch. Dermatol. Syph. Volume 138, 1922, pp. 252-257.
- ↑ Ernest Finger: The side effects of salvarsan. In: Wiener medical Wochenschrift. Volume 62, 1911, pp. 2701-2708.
- ↑ Karl Bohac, Paul Sobotka: About undesirable side effects after using Dioxydiaminoarsenobenzol (606) Ehrlich-Hata. In: Wiener Klinische Wochenschrift. Volume 23, 1910, pp. 1099-1102; see. Paul Ehrlich: About bladder disorders after using the preparation 606. Reply to the article by Dr. Karl Bohac and Doctor Paul Sobotka, assistants at the dermatological clinic in Prague in No. 30 of this magazine. In: Wiener Klinische Wochenschrift. Volume 23, 1910, p. 113.
- ↑ Paul Tachau: Salvarsan side effects. Critical overview. Hall 1923.
- ↑ EM Lewin: About experiments in the field of Salvarsanikterus. XII. Communication on the effects of salvarsan on the liver. In: Arch. Dermatol. Syph. Volume 166, 1932, pp. 716-721. Same thing: News on the doctrine of the toxic effects of arsenobenzene preparations on the liver. Ibid, Volume 167, 1933, pp. 481-486.
- ↑ Gerd Peters : On the pathology, pathogenesis and clinic of salvarsan damage to the central nervous system. In: Neurologist. Volume 18, 1947, pp. 66-71.
- ↑ Florian G. Mildenberger (2012/13), pp. 334 f., 337 f. and (particularly on Salvarsan deaths up to 1918) 349–361.
- ↑ Erich Hoffmann : Advances in the recognition and treatment of syphilis. Long-term success of the combined mercury-salvarsan treatment. Bonn 1913.
- ↑ Harald Boas: Two cases of reinfection in patients treated with salvarsan mercury, together with a compilation of our results with the combined treatment. In: Münchner medical Wochenschrift. Volume 60, 1913, p. 2620 f.
- ↑ Steven Riethmiller: From Atoxyl to Salvarsan. Searching for the magic bullet. In: Chemotherapy. Volume 51, 2005, pp. 234-242.
- ↑ See also Paul de Kruif : Paul Ehrlich. The magic ball - the Salvarsan. In: Paul de Kruif: Microbe hunters. (Original edition: Microbe Hunters. Harcourt, Brace & Co., New York 1926) Orell Füssli Verlag, Zurich / Leipzig 1927; 8th edition ibid 1940, pp. 324-346.
- ↑ See also Steven Riethmiller: From Atoxyl to Salvarsan. Searching for the magic bullet. In: Chemotherapy. Volume 51, 2005, pp. 234-242.
- ^ Entry on Salvarsan. In: Römpp Online . Georg Thieme Verlag, accessed on November 16, 2014.
- ↑ Axel Hüntemann: Hygiene on behalf of the state. The Reich Health Office 1876–1933. Göttingen 2008, p. 255.
- ↑ Rudolf Kafeman: syphilis prevention or Salvarsan? Munich 1915, p. 8.
- ↑ Harald Boas: Can a positive seroreaction in syphilis turn out to be negative? In: Negotiations of the German Dermatological Society, 17th Congress held in Berlin 8. – 10. October 1935. In: Arch. Dermatol. Syphilis. Volume 172, 1935, p. 57.
- ↑ W. Gahlen : The limits of the normal when the seroreactions decrease after syphilis treatment. In: dermatologist. Volume 4, 1953, pp. 380-384.
- ↑ Heinrich Dreuw: About the evaluation of the Wassermann reaction. In: German Medical Weekly . Volume 36, 1910, pp. 166-169.
- ↑ Carl Bruhns : About unconscious late syphilis together with reports on failure of the Wassermann reaction in 1800 people allegedly not infected with syphilis (Berl. Klin. Wochenschr. 1916, No. 30). In: Allg. med. Centr.-Ztg. Volume 85, 1916, No. 41, p. 163.
- ↑ Gustav Emanuel: Influence of the Wassermann reaction of the normal rabbit by mercury and Salvarsan. In: Berlin clinical weekly. Volume 69, 1921, p. 197 f.
- ^ Rudolf Krefting: Syphilis treatment exclusively with Salvarsan. In: German Medical Weekly . Volume 41, 1916, pp. 978-981.
- ↑ Elke Tashiro: The scales of Venus. Venereological experiments on between progress and morality. Husum 1991, p. 120.
- ↑ Heinrich Dreuw: Wassermann reaction and prostitutes investigation. In: German Medical Weekly . Volume 37, 1911, p. 1482 f.
- ↑ Heinrich Dreuw: The Salvarsangefahr. Berlin 1914.
- ^ An unsuccessful Salvarsand debate. In: Naturarzt. Volume 46, 1918, p. 39.
- ^ Oskar Mummert: On the upcoming Salvarsand debate in the House of Representatives. In: Naturarzt. Volume 45, 1917, p. 208.
- ^ The Salvarsan in front of the House of Representatives. Speech by Member Haehnisch, given in the Prussian House of Representatives on March 1, 1917 (end). In: Archive for physical-dietary therapy in medical practice. Volume 19, 1917, pp. 213-218.
- ^ Oskar Mummert: Dr. Dreuw and the Ministry of Science etc. In: Naturarzt. Volume 48, 1920, pp. 157-159.
- ↑ Ake Liljenstrand: Assay of the curative action of neoarsphenamine by the time-mortality data. In: Journal of Pharmacy and Pharmacology. Volume 1, 1949, pp. 78-86.
- ↑ Victor Mentberger: Development and current status of arsenic therapy for syphilis with special consideration of salvarsan (Ehrlich-Hata 606) and neosalvarsan. In addition to a systematic compilation of the literature published so far. Jena 1913; see. on this: Julius Benario: Critical remarks on Mentberger's compilation of the Salvarsan and Neosalvarsan deaths. In: German Medical Weekly . Volume 40, 1914, pp. 1262-1265.
- ↑ Rudolf Matzenauer: The prognosis of syphilis. In: E. Finger u. a. (Ed.): Handbook of sexually transmitted diseases. Volume 3, part 3. Vienna / Leipzig 1916, pp. 2458–2481, here: p. 2625.
- ^ Karl F. Goetz: Salvarsan deaths with special consideration of the silver Salvarsan. Medical dissertation Munich 1921, especially p. 14.
- ↑ See also Walther Schönfeld : About the one-time combined intravenous mercury salvarsan treatment of syphilis with special consideration of Novasurol silver salvarsan mixtures. In: Munich medical weekly. Volume 68, 1921, pp. 197-199.
- ↑ Peter Gienow: The miasmatic therapy of syphilina. 2nd Edition. Peter Irl, 2007, p. 132 ( Google Books ).
- ^ Eugen Galewsky : 2 years of silver salvarsan therapy. In: Munich medical weekly. Volume 67, 1920, pp. 124-127.
- ↑ Florian G. Mildenberger (2012/2013), pp. 338–344 ( The Debate 1913/1914 ) and 344–352.
- ↑ Florian G. Mildenberger: No salvation through arsenic? The salvarsand debate and its consequences. 2012/13, pp. 327, 358 and 364-369.
- ↑ Rudolf Pedestrian: Spirotrypan (A new arsenobenzene preparation). In: dermatologist. Volume 2, 1951, pp. 413-417.
- ^ Wolf-Helmut Wagner , Willy Schulz: Chemotherapeutic studies on Spirotrypan. In: Zschr. Ges. exp. Med. Vol. 119, 1952, pp. 204-228.
- ↑ Klemens Thelen: Clinical-serological experience in the treatment of syphilis with Spirotrypan. In: Journal of Skin and Venereal Diseases. Volume 15, 1953, pp. 126-130.
- ↑ G. Weber, U. Falk: Catamnesis of a Lues connata tarda treated with "Ehrlich 606". In: dermatologist. Volume 22, 1971, pp. 521-523.
- ↑ KH Lawrenz: Spirotrypantherapie at connataler syphilis. In: Pediatric Practice. Volume 22, 1954, pp. 248-252.
- ↑ Florian G. Mildenberger (2012/13), p. 365 and 368.
- ↑ Florian G. Mildenberger (2012/2013), p. 372.
- ^ A b Nicholas C. Lloyd, Hugh W. Morgan, Brian K. Nicholson, Ron S. Ronimus: The composition of Ehrlich's Salvarsan: Resolution of a century-old debate. In: Angewandte Chemie, International Edition. Engl. Vol. 44, No. 6, 2005, pp. 941-944. PMID 15624113 , doi: 10.1002 / anie.200461471 .
- ^ AL Rheingold, PJ Sullivan: Crystal and molecular structure of hexaphenylcyclohexaarsine, cyclo- (AsPh) 6. In: Organometallics . Volume 2, No. 2, 1983, pp. 327-331, doi: 10.1021 / om00074a021 .