Azo dye
Azo dyes are numerically the largest group of synthetic dyes . One or more azo bridges (–N = N–) as part of the chromophore are characteristic of azo dyes . Azo dyes with several azo groups are referred to as bisazo (also disazo), trisazo, tetrakisazo, polyazo dyes. Bisazo dyes which are produced by coupling a diazonium compound with a bifunctional coupling component or by reacting a bisdiazonium salt with a coupling component are referred to as primary disazo dyes. Secondary bisazo dyes are obtained by further diazotizing and coupling an amino group-containing monoazo compound.
CI Acid Orange 36 : monoazo dye from metanilic acid as diazo and diphenylamine as coupling component.
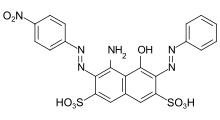
CI Acid Black 1 : primary bisazo dye made from a bifunctional coupling component ( H-acid ) and two different diazo components ( aniline and p -nitroaniline ).
CI Direct Blue 8 : primary bisazo dye made from a bisdiazonium salt ( 3,3'-dimethoxybenzidine ) and Neville-Winther acid as a coupling component
CI Solvent Red 26 : secondary bisazo dye. o -Toluidine is diazotized and coupled to 2,5-xylidine . The monoazo dye is further diazodized and coupled to 2-naphthol .
In contrast to the azo pigments , the azo dyes are soluble in the respective application medium.
history
In the middle of the 19th century, industrialization had spread from England to all of Western and Central Europe. The textile industry received an upswing through the mechanization of the spinning and weaving process, which also increased the demand for dyes. The production of naturally obtained dyes did not meet these new requirements, which promoted color research and the rapid development of the chemical industry.
In 1844 Justus von Liebig ventured the prognosis that methods would soon be discovered to synthetically manufacture dyes and medicinal substances from coal tar .
In 1856, while experimenting with coal tar , the Englishman William H. Perkin succeeded in synthesizing the first artificial tar dye, mauvein , which, however, is not an azo dye. At the London World's Fair, however, he caused a stir, which also promoted further research. The diazotization was then found in 1857 by Peter Grieß , a student of August Wilhelm von Hofmann . Chemists all over Europe discovered manufacturing routes for a whole range of such tar dyes , from Hofmann violet to fuchsine , from aniline yellow (1861) to Bismarck brown , the latter two being important representatives of the group of azo dyes. The starting material was aniline, which was obtained from coal tar, which is why this group was also called tar pigments . Aniline yellow is listed in the Color Index as CI Solvent Yellow 1 , a dye that is not water- but fat-soluble . Congo red ( CI Direct Red 28 ) was produced in 1884 as the first disazo dye (also known as disazo dye ). CI Disperse Yellow 8 was developed in 1926 as a water-insoluble disperse dye and is still used today for dyeing polyester fabrics.
Azo dyes were used as food dyes at an early stage , for example butter yellow (CI Solvent Yellow 2) for coloring butter and margarine. However, the use of butter yellow to color margarine was banned in 1938 after it was discovered that it could cause liver cancer in rats. In addition, around 1949 there were only a few restrictions on adding azo dyes to foods for “beautification”.
Structure and properties
Azo dyes are numerically the largest class of dyes . They are characterized by the general formula R 1 -N = NR 2 . The two radicals (R 1 and R 2 , usually aromatic) can be identical or different.
The azo group -N = N- with the chromophoric nitrogen double bond is typical of azo dyes . These synthetic dyes use aromatic amines as a starting material , in the simplest case aniline. Azo dyes achieve their diversity through the simple substitution of the hydrogen atoms on the aromatic rings , which influence the chromophoric system auxochrome and which can be used to control the color shade.
Azo dyes often have polar and non-polar substituents and can thus be tailored to the required medium. With the appropriate structure, they can also form hydrogen bonds in addition to van der Waals bonds .
Representatives of the group are color stable and produce lightfast and strong colorations. With a suitable constitution, they can be wash-, dry-clean and rub-fast on textiles.
At room temperature in the dark, the trans configuration of stable aromatic azo compounds is the energetically most favorable conformation. When exposed to light or heat, this conformation to the cis isomer and thus its geometric shape can change. This can be a useful property for making reversible molecular switches. By introducing fragments of stable azo compounds such as azobenzene into biologically active molecules such as proteins, a multitude of biological processes can be controlled in space and time by irradiation with light instead of reagents.
Manufacturing
The variety of azo dyes results from the comparatively simple production method. The diazotization of aromatic amines gives diazonium salts which react with electron-rich aromatic compounds or β-dicarbonyl compounds in the sense of an azo coupling .
The synthesis takes place in two steps:
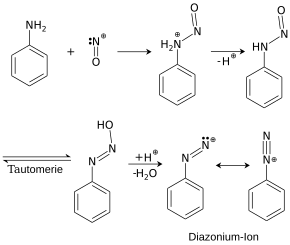
1. Diazotization : The solution or suspension of an aromatic amine is acidified with hydrochloric or sulfuric acid and a sodium nitrite solution is added at low temperature . An electrophilic nitrosyl cation (NO + ) is formed.
Formation of the nitrosyl cation
The nitrosyl cation is attacked by the lone pair of electrons on the nitrogen atom on the arylamine. When a proton (H + ) is split off , an N-nitroso compound (Ar-NH-N = O) is formed, which rearranges into the unstable aryldiazohydroxide (Ar-N = N-OH). This decomposes with the splitting off of an OH - ion and an aryldiazonium ion (Ar-N 2 + ) is formed. During diazotization, the temperature should always be below 5 ° C, as otherwise the diazonium salts will split off nitrogen (risk of decomposition).
Diazotization of aniline
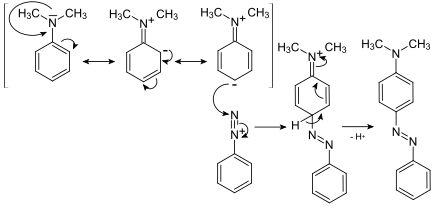
2. Azo coupling : The azo coupling is an electrophilic second substitution . The substituents that have a + M effect increase the electron density and thus the reactivity. In addition, they direct the secondary substituents in the para position. The ortho position is rare for reasons of steric hindrance .
Azo coupling of anililine diazonium salt with N, N -dimethylaniline to butter yellow (CI Solvent Yellow 2)
use
This group of dyes is represented in the entire color range due to the wide range of coupling possibilities with dyes. Azo dyes are used to color textiles, fats and oils, to color wax, straw, wood and paper. They are also used for coating materials, such as CD-Rs . Azo dyes, which can release toxic or carcinogenic amines , are banned in Germany for everyday objects and tattoo inks. They may not be used for dyeing fabrics or for jewelry and under no circumstances for cosmetics .
Selected azo dyes have been tested and approved for their suitability as food coloring. Incidentally, such dyes have several sulfonic groups in order to increase the solubility in water. The high water solubility instead of fat solubility of the color body prevents the risk of storage in the body, as the substances are more easily excreted through the urine.
Azo dyes are also used for leather dyes, whereby attention is paid to liposolubility, but coupling products with certain amines may not be used here either.
There were health concerns, among other things, with Easter eggs dyed with azo colors, which, however, remained unconfirmed because a much too low dose of the dye got through the shell into the egg.
In medicine, selected products are used to stain cancer cells. They are also important in clinical chemistry to detect certain metabolic products such as bilirubin . They arise from complex formation between various reagents and the substance to be detected and are then measured photometrically.
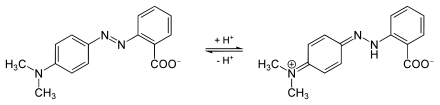
The azo bridge is in protonated or deprotonated form, depending on the pH value . This is associated with a shift in color depth. Azo dyes are therefore also used as acid-base indicators . Examples include methyl , methyl orange , Congo Red and Alizarin Yellow R . In addition, there are also redox indicators among the azo dyes.
Methyl red is colored yellow in the neutral (left structure), so it absorbs violet light. At a pH value below 4.4, the molecule is protonated on the nitrogen, the polarized azo bridge leads to a deepening of color to red through the absorption of now green (longer-wave) light (right structure).
Health hazard
It has been proven that the human body is able to break down the azo dyes absorbed at the azo bridge back into the starting materials through reductive cleavage. This can be done by intestinal bacteria, by azoreductases of the liver or by extrahepatic tissue. It is therefore suspected that all azo dyes that contain a releasable carcinogenic arylamine component have a carcinogenic potential.
Azo dyes that are built up from at least one of these carcinogenic amines are prohibited in textiles and leather in Germany according to the Consumer Goods Ordinance (BedGgstV). EU-wide, these are prohibited in consumer products according to the REACH regulation . This prohibition applies to textiles and leather that can come into direct contact with human skin or the oral cavity for a long period of time. Currently, 24 aromatic amines are affected, the best known of which is benzidine . Benzidine dyes are suspected of significantly increasing the risk of bladder cancer when exposed . According to the EU directive, azo dyes that can release such amines with more than 30 ppm in the finished product through reductive cleavage of azo groups may not be used and corresponding textile and leather products may not be placed on the market. The analytical limit of quantification varies by 5 ppm depending on the amine. In the EU, these dyes have not been used in the textile and leather industry for years. Since imports of consumer goods (such as textiles from Asia) may not be dyed with such harmful dyes, the competent authorities carry out random checks.
In contrast to azo dyes, azo pigments are practically insoluble in the application medium. Because of their insolubility, azo pigments are not bioavailable and therefore non-toxic and non-carcinogenic. They are used for printing inks, plastics, lacquers, toners and food packaging.
Legal situation
On March 25, 2010, the EU Commission commissioned the EFSA (European Food Safety Authority) to carry out a new health assessment of questionable azo dyes. Since July 20, 2010, foods containing the azo dyes tartrazine (E 102), yellow orange S (E 110), azorubine (E 122), allura red (E 129) or cochineal red A (E 124) must be included in the European Union the separate warning “May impair the activity and attention of children”.
literature
- Agnes Slowicki, Heiko U. Käfferlein, Thomas Brüning: Skin permeability of azo colorants. Part 1: Properties, skin absorption and metabolism. Hazardous substances - keeping the air clean 69 (6), pp. 263–268 (2009).
Web links
- Directive 2002/61 / EC
- More detailed information on the “folding electron pairs” during the azo coupling
- Further information on the diazotization mechanism
Individual evidence
- ^ Rudolf Winderlich: Textbook of Chemistry for Higher Educational Institutions: Standard edition for lower and upper grades . Springer-Verlag, 2013, ISBN 978-3-663-04370-6 , pp. 391 ( books.google.de ).
- ^ Heinz-Gerhard Franck, Jürgen W. Stadelhofer: Industrial Aromatic Chemistry: Raw Materials · Processes · Products . Springer-Verlag, 2013, ISBN 978-3-662-07875-4 , pp. 6 ( books.google.de ).
- ↑ Werner Baltes: Food chemistry . 5th edition. Springer Verlag, Berlin, Heidelberg 2000, ISBN 978-3-662-08282-9 , pp. 187 ( limited preview in Google Book search).
- ↑ Ernst Kern : Seeing - Thinking - Acting of a surgeon in the 20th century. ecomed, Landsberg am Lech 2000, ISBN 3-609-20149-5 , p. 198.
- ↑ Estíbaliz Merino, Maria Ribagorda: Control over molecular motion using the - photoisomerization of the azo group . In: Beilstein Journal of Organic Chemistry . tape 8 , 2012, p. 1071-1090 , doi : 10.3762 / bjoc.8.119 .
- ^ Ned A. Porter, Lawrence J. Marnett: Photolysis of unsymmetric azo compounds. cis Azo compound intermediates . In: Journal of the American Chemical Society . tape 95 , no. 13 , June 27, 1973, pp. 4361-4367 , doi : 10.1021 / ja00794a036 .
- ↑ Michael B. Smith, Jerry March: March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure . John Wiley & Sons, 2007, ISBN 978-0-470-08494-6 , pp. 346 ( books.google.de ).
- ↑ Easter egg colors - Cancer on installments? April 8, 2012, Jochen Kleboth, lebensmittelfokus.at food focus ( Memento of 11 December 2013, Internet Archive )
- ↑ Consumer Goods Ordinance, Appendix 1 (to Section 3). Substances that may not be used in the manufacture or treatment of certain commodities. Federal Ministry of Justice and Consumer Protection, accessed on October 29, 2019 .
- ↑ Regulation (EC) No. 1333/2008 of the European Parliament and of the Council of December 16, 2008 on food additives. (PDF) .
- ↑ Reinhard Wolff: Brightly colored is unhealthy . Article in the daily newspaper of July 19, 2010, p. 9.
- ↑ Foodwatch : Warning for azo dyes . Press release from July 20, 2010.