Methyl orange
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Methyl orange | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 14 H 14 N 3 NaO 3 S | ||||||||||||||||||
| Brief description |
orange solid with a faint odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 327.34 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.28 g cm −3 |
||||||||||||||||||
| Melting point |
> 300 ° C |
||||||||||||||||||
| solubility |
poor in water (approx. 5 g l −1 at 20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Methyl orange is an azo dye from the application group of acid dyes . It is present as the sodium salt of 4- [4- (dimethylamino) phenylazo] benzenesulfonic acid and is mostly used as a pH indicator .
presentation
Sulphanilic acid is diazotized with nitrous acid :
The diazonium salt is then coupled with N , N -dimethylaniline ( green ), converted into the sodium salt with sodium hydroxide solution and precipitated :
properties
Methyl orange forms orange crystals.
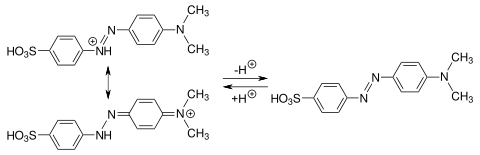
In the strongly acidic pH range, the azo group is protonated and a mesomeric quinoid system is obtained . When base is added, the group deprotonates in the pH range 3.0 to 4.4 and the color changes from red to yellow-orange.
Web links
Individual evidence
- ↑ a b c d e Entry on sodium 4- (4-dimethylaminophenylazo) benzenesulfonate in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ Applicher: Safety data sheet methyl orange (CI 13025) ( Memento of the original from September 16, 2016 in the Internet Archive ) Info: The archive link has been inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. As of August 5, 2016, accessed on September 12, 2016
- ↑ Data sheet methyl orange (PDF) from Merck , accessed December 9, 2010.
- ^ Author collective: Organikum . 21st edition. Wiley-VCH, 2001, ISBN 3-527-29985-8 , p. 645.
- ↑ Indicator Reagents . In: Ullmann's Encyclopedia of Industrial Chemistry (engl.).