Quinones
Quinones ( English Quinones ) are a group of organic compounds for which it is crossed cyclic conjugated diketones is. They are oxidation products of dihydroxy aromatics . The two simplest representatives are 1,2- and 1,4-benzoquinone , which are formed when the two dihydroxybenzenes pyrocatechol and hydroquinone are oxidized and the aromaticity of the ring is eliminated . 1,4-Benzoquinone is sometimes also referred to synonymously as quinone.
The name quinone is derived from quinic acid , whose oxidation u. a. leads to 1,4-benzoquinone. The nomenclature of quinones is based on the aromatic stem skeleton (benzo-, naphtho-, anthra-), prefixes the positions of the two quinoid carbonyl groups in the molecule (e.g. 1,2-, 1,4-) and ends with “Quinone” (examples: 1,4-benzoquinone, 1,2-naphthoquinone , 9,10-anthraquinone ). Compounds whose chemical structure contains a quinone element are called quinoid , and the structural element itself is called the quinoid system . The reduction of quinones produces the associated hydroxyaromatics, which are also called quinoles .
The quinones, which are all colored, are oxidizing agents whose redox potential is significantly changed by substituents (halogen, cyano, alkyl, hydroxyl groups, etc.). A distinction is made between 1,2 ( ortho ) quinones (e.g. pyrroloquinoline quinone ) and 1,4 ( para ) quinones (e.g. anthraquinone ).
Formal replacement of the oxygen of a quinoid carbonyl group by = NH, = NOH, = N 2 or = CH 2 leads to quinone imines , quinone oximes, quinonediazides and quinone methides .
There are many poisons among the quinones , but also a wide range of vital substances, e.g. B. Coenzyme Q10 (coenzyme Q 10 ), Vitamin K and pyrroloquinoline quinone .
Quinone was first synthesized in 1838 by the Russian Liebig student Alexander Abramowitsch Voskressenski .
Occurrence
In nature, quinones are particularly common in dyes, e.g. B. in fungi , bacteria or flowers . Chinoid systems are also found in various antibiotics . Among other things, quinones are formed by the enzymatic oxidation of polyphenols. For example, they play an important role in the browning of cut apples .
Well-known quinones are:
- the ubiquinones as electron carriers in the respiratory chain
- the plastoquinones in photosynthesis
- the K vitamins ( phylloquinone , menaquinone )
- the pyrroloquinoline quinone (PQQ)
- the Tryptophantryptophylchinon (TQQ)
- the dichlorodicyanoquinone (DDQ)
- the hydroxynaphthoquinones (in henna and walnuts )
- Echinochrome A, adrenochrome, dopachrome
Quinones are also found in the defensive secretions of insects, especially benzo- and toluquinones .
presentation
Quinones form u. a. by oxidation of phenols or polycyclic aromatic hydrocarbons . Cer (IV) compounds such as ammonium cerium (IV) nitrate prove to be particularly useful.
Redox-active and reactive o- quinones can be produced biosynthetically by converting trans -dihydrodiols with the aid of the enzyme AKR1A1 .
Reactions
Are synthetically important (if any photochemically induced) cycloaddition reactions of quinones to cyclobutane derivatives, oxetanes , cage compounds and pyrans can lead. Olefins add z. B. on exposure to the 1,2-dicarbonyl system of ortho -quinones, which in these cases acts as a hetero-1,3-diene system (see Diels-Alder reaction ). This takes place, among other things, with the formation of dioxane derivatives ( Schönberg reaction ).
In contrast to the pure substances, the deeply colored 1: 1 mixture of 1,4-hydroquinone and 1,4-benzoquinone is called quinhydrone , a classic example of a charge transfer complex .
Semiquinons
The reduction of quinones to quinoles takes place in several stages via reactive radical intermediate compounds, the semiquinones.
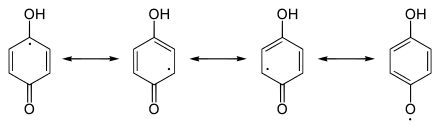
The reduction processes are coupled with acid-base equilibria. These are e.g. Sometimes quite consistent, like the semiquinones of the ubiquinones . The reduction of quinone with the formation of a semiquinone and its further reduction to hydroquinone is explained using the example of 1,4-benzoquinone :
After protonation and reduction, the 1,4-benzoquinone 1 forms the semiquinone 2 . The unpaired electron is distributed over the semichinoid system. Further reduction along with protonation leads to hydroquinone 3 .
-

Redox equilibria (horizontal) and acid-base equilibria (vertical) for quinone using
the example of 1,4-benzoquinone.
use
Quinones are used as oxidizing agents, bactericides and analytical reagents. They also arise as an intermediate product in the synthesis of dyes (see also alizarin ). Quinones also play an important role in anti-malarial drugs . In technical chemistry they are especially important for the production of hydrogen peroxide via anthraquinone.
Individual evidence
- ^ A b Eberhard Breitmeier, Günther Jung: Organic chemistry . Basics, substance classes, reactions, concepts, molecular structure. 5th edition. Georg Thieme Verlag, Stuttgart 2005, ISBN 3-13-541505-8 , p. 355 ff . ( limited preview in Google Book search).
- ↑ Winfried R. Pötsch, Annelore Fischer and Wolfgang Müller with the assistance of Heinz Cassebaum: Lexicon of important chemists , VEB Bibliographisches Institut Leipzig, 1988, ISBN 3-323-00185-0 , p. 441.
- ^ Nisha T. Palackal, Michael E. Burczynski, Ronald G. Harvey, Trevor M. Penning: The Ubiquitous Aldehyde Reductase (AKR1A1) Oxidizes Proximate Carcinogen trans-Dihydrodiols to o-Quinones: Potential Role in Polycyclic Aromatic Hydrocarbon Activation. In: Biochemistry. Volume 40, 2001, p. 10901, doi : 10.1021 / bi010872t .
- ^ L. Michaelis, MP Schubert: The Theory of Reversible Two-step Oxidation Involving Free Radicals. In: Chem. Reviews. 22 (3), 1938, pp. 437-470; doi: 10.1021 / cr60073a003 .
- ↑ after Paul Rys, Heinrich Zollinger: dye chemistry. A guide . 3., rework. Edition. Wiley-VCH, 1982, ISBN 3-527-25964-3 , p. 45.