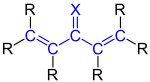
Cross conjugation
| Principle of cross-conjugated double bonds |
| A cross-conjugated triene, R is an organyl radical (e.g. alkyl radical) or a hydrogen atom. |
| A cross-conjugated ketone (X = O) or imine (X = NR). R is an organyl radical (e.g. alkyl radical) or a hydrogen atom. |
As a cross-conjugation (English. Cross-conjugation ) is a special type of in organic chemistry conjugation of double bonds , respectively. To do this, there must be at least three double bonds linked to one another via single bonds. One of them must have a fork-shaped branch (see examples below). This is the "classic" definition. Another is based on a special MO theory (PMO method according to Dewar).
Cross-conjugated double bond systems
After clarity about the constitution of the most important "unsaturated" compounds had been achieved in organic chemistry towards the end of the 19th century , it remained a mystery how the constitution and color of organic substances were connected. So was z. B. 1,4-Benzoquinone or Phoron yellow, while other ketones such as acetone or acetophenone showed no color.
In 1900 Johannes Thiele reported on reactions of cyclopentadiene with various ketones , which produced unsaturated hydrocarbons, which he called fulvene (Latin: fulvus , brown-yellow, red-yellow) "because of their bright color" . Thiele saw this as proof "that the coloring of organic compounds is essentially due to the way in which the double bonds are arranged."
For this special arrangement of the double bonds, the term “cross-conjugated” has become common. The term was and is attributed to Thiele. However, the word “crossed” does not appear in his communication. It can be found in contemporary literature from Hermann Staudinger and Auers.
Since then, numerous molecules with the structural element “cross-conjugated double bond” have been discovered and synthesized.
An explanation of the connection between constitution and color only became possible in the 20th century through quantum chemistry . It has been recognized that it is misleading to compare the color of fulvene and unsaturated carbonyl compounds; because this results from different electronic transitions (electronically excited states), namely an n-π * transition (quinone) or π-π * transition (fulvene).
Effects of cross-conjugated double bonds
Does the presence of cross-conjugated double bonds in a molecule cause observable effects, and if so, which?
To explain the color, more precisely: the absorption of visible or ultraviolet light (UV / VIS spectrum, electron spectrum), the term cross-conjugation should be dispensable today.
Does cross-conjugation have a significant influence on the structure of organic compounds, i. H. Warmth of formation, bond lengths, bond angles or dipole moments?
In 1968 Phelan and Orchin presented a paper in which they examined the hydrocarbon 3-methylene-1,4-pentadiene under the aspect of the VB theory and the Hückel approximation (HMO model) available at the time in the MO theory . This unsaturated hydrocarbon can be viewed as a prototype of the cross-conjugated systems, it is, so to speak, an “open fulvene” (Staudinger). However, different conformations are possible here, between which no distinction is possible in the HMO approximation.
Phelan and Orchin found that the Hückel resonance energy is 0.900 β and the bond order of the C-1 / C-2 bond is higher than that of the reference molecule 1,3-butadiene . For the bond C-3 / C-6 (branching) the “double bond character” should be less than for 1,3-butadiene. The authors came to the conclusion: "... in the ground state there is some net bonding or conjugation between the conjugated centers."
Experimental data to verify this prediction do not appear to be available. The molecule is very unstable.
Phelan and Orchin extended the concept of cross-conjugation to include other compounds, even urea . In this case, the “free” (non-binding) electron pairs on the nitrogen atoms are regarded as equivalents of a C = C π bond. It remains to be seen how useful this extension is.
As far as the “cross-conjugated” carbonyl compounds are concerned, the HMO calculations showed that, on the whole, the extent of the electronic interaction with the second C = C double bond is small at best.
Today we know the limits of HMO considerations, and the question arises whether the results of this theory are in agreement with the experiment. Until this is clear, the term “cross conjugation” should be used with great caution. It dates from a time before the development of the quantum chemical theories of chemical bonding.
Individual evidence
- ↑ MJS Dewar, Color and constitution. Part III. Polyphenyls, polyenes, and phenylpolynes; and the significance of cross-conjugation, J. Chem. Soc. , 1952, 3544-3550, doi : 10.1039 / JR9520003544 .
- ↑ Hugo Kauffmann, Ueber the relationship between color and constitution in chemical compounds, in: Collection of chemical and chemical-technical lectures (Ed. Felix B. Ahrens), Vol. 9, Stuttgart, Ferdinand Enke, 1904. This work gives a good overview about the beliefs at the beginning of the 20th century.
- ^ J. Thiele, Reports of the German Chemical Society , 33, 666-673 (1900).
- ^ H. Staudinger, Reports of the German Chemical Society, 41, 1493-1500 (1908), Reports of the German Chemical Society 42, 4249-4262 (1909).
- ↑ K. Auers, F. Eisenlohr, J. Prakt. Chem. 84, 37-121 (1911).
- ↑ Nelson F. Phelan and Milton Orchin, Cross Conjugation, J. Chem. Educ. 45: 633-637 (1968).
- ^ Reports of the German Chemical Society, 41, 1493–1500 (1908).