Hückel approximation
The Hückel approximation proposed by Erich Hückel , also: Hückel molecular orbital model / method (HMO model / method), is a method of semiempirical quantum chemistry that delivers surprisingly good results with little computational effort. It allows to approximate molecular orbitals in conjugated π systems. The two most important consequences are the Hückel rule and the Woodward-Hoffmann rules .
application
The approximation is derived from Ritz's method using a basis of atomic p orbitals. The eigenvalue problem is formally reduced to . In the first case is an eigenfunction of the molecule Hamilton operator . In order to determine it exactly, a 6n -dimensional partial differential equation would have to be solved for an n -atomic molecule , which is generally not possible analytically. In the second case is the n -dimensional vector that contains the coefficients for linear combination.
After it has been calculated, the molecular orbital can be given as a corresponding linear combination of the individual p z orbitals. The value E represents the energy of the orbital.
In the Ritz method, the best possible solution is found iteratively using the Hartree-Fock method . For this, a large number of integrals have to be solved in each step, which means a high computational effort. The simplification of the Hückel approximation is that all integrals are parameterized.
The n conjugated atoms in the molecule are numbered consecutively. The matrix is an n × n matrix. One sets:
h ii = α i
h ij = β , if the two atoms are adjacent (and linked by conjugation), and
h ij = 0 otherwise.
α i is the Coulomb integral of the atom i in the molecule ( α i <0 )
(* is the complex conjugation, V denotes the entire volume)
β is the resonance integral between two atoms i , j (is assumed to be the same for all atom pairs) ( β <0 )
The point of the approximation is that these two integrals are not calculated. For example, they can be estimated using empirical data. For two atoms of the same kind the Coulomb integrals are equated. It is therefore particularly easy to calculate pure hydrocarbons. Only two constants α and β remain. The eigenvectors are independent of their value.
Derivation of the Hückel rule
The Frost circle offers a simple way of estimating the stability of cyclically conjugated planar molecules. It is based on the surprising fact that the energy level scheme of a cyclically conjugated planar n-atomic molecule can be represented as a regular n corner (provided that the planar molecule has an n -fold main axis of rotation).
The a n ring corresponding n × n matrix is formed by in the main diagonal α writes, into the two secondary diagonals β and also in the lower left and the upper right corner of β :
The eigenvalues of this matrix give to him . If you now apply the energy along the y -axis, note that β is negative and keep the corresponding distances in the x -direction, you get an n -gone standing on top .
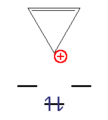
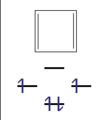
- Examples of aromatic compounds
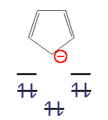
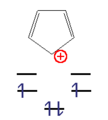
This n corner can now be filled with an odd number of electron pairs ( 4n + 2 π electrons) with a high gain in energy. These molecules are called " aromatics ". With an even number of electron pairs ( 4n π-electrons), the two uppermost levels would be half-occupied according to Hund's rule , the molecule would be paramagnetic and the total energy gain would be small. These compounds are unstable and are called "anti-aromatics".
- Examples of anti-aromatic compounds
example
benzene
The Hückel matrix is:
The eigenvalues result from:
The eigenvectors for the respective eigenvalues:
((1, 1, 1, 1, 1, 1), (1, 0, -1, -1, 0, 1), (-1, -1, 0, 1, 1, 0), (-1 , 1, 0, -1, 1, 0), (-1, 0, 1, -1, 0, 1), (-1, 1, -1, 1, -1, 1))
A section (parallel to the xy plane at a distance of 75 pm) through the corresponding orbitals is shown on the right. The coordinates of the vectors mean the sign of the p z wave functions. These are counted counterclockwise starting from the right.
According to a hexagon standing on the tip, there are 3 stabilized and three destabilized orbitals. 6 electrons can occupy the lower three orbitals with a high gain in energy. The total binding energy is 2 · 2 β + 4 β = 8 β . This value is significantly higher than 6 β for three isolated π bonds.
swell
- Peter W. Atkins: Physical Chemistry . Weinheim [u. a.]: VCH-Verl.-Ges.
- Dietmar Dorninger: Mathematical basics for chemists . Eisenstadt: Prugg
literature
G. Frenking: Perspective on “Quantum theoretical contributions to the benzene problem. I. The Electronic Configuration of Benzene and Related Relations ” . In: Theoretical Chemistry Accounts . tape 103 , 2000, pp. 187-189 , doi : 10.1007 / s002149900023 .