Woodward-Hoffmann rules
The Woodward-Hoffmann rules developed by Robert B. Woodward and Roald Hoffmann in 1965 allow statements to be made about the course and products of pericyclic reactions . They include electrocyclic reactions , sigmatropic rearrangements, and cycloadditions . The rules take into account that the reaction can be either thermal or photochemical. Hoffmann was honored with the Nobel Prize in Chemistry in 1981 together with Fukui Kenichi for this work .
rule
The Woodward-Hoffmann rule can be expressed quite simply:
- If the reactions proceed in concert, the orbital symmetry is retained.
The Woodward-Hoffmann rules apply to concerted reactions involving π orbitals . They do not apply to reactions that take place via reactive intermediates or via radical mechanisms .
The importance of the Woodward-Hoffmann rules is that they not only consider the π orbitals, but also take into account their signs during the course of the reaction. In principle, binding interactions, and thus a later chemical bond, are only possible between orbital lobes with the same sign.
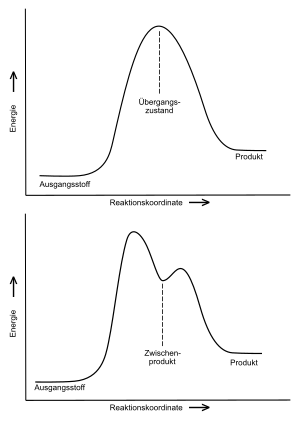
Concerted reactions
Concerted reactions are characterized by the fact that they take place in one step. The loosening of the old and the tying of the new binding takes place in a single step, but not necessarily synchronously. Accordingly, there are no intermediates in concerted reactions , only transition states . An intermediate product has a local energy minimum along the reaction coordinate, whereas the transition state has an energy maximum. Intermediate products can usually be trapped and isolated, transition states cannot.
Cycloadditions
Two molecules each with a π system react to form a new cycle ( ring closure ).
Electrocyclic reactions
At the end of a linear system of π electrons, a σ bond is formed, the π system is shortened by a π bond (electrocyclic ring closure) or vice versa:
At the end of a cyclic system of π electrons, interrupted by three σ bonds, the middle σ bond is broken and the π system is lengthened by one π bond (electrocyclic ring opening).
Sigmatropic rearrangement
Sigmatropic rearrangements are a special case of a pericyclic reaction. The compound's electronic system is rearranged. A substituent bound to the system with a σ bond migrates along the π system.
Special rules
The rules for electrocyclic reactions are as follows:
- Even number of conjugated π-bonds:
- thermal reaction → conrotatory
- photochemical reaction → disrotatory
- Odd number of conjugated π-bonds:
- thermal reaction → disrotatory
- photochemical reaction → conrotatory
Con- / disrotatory rotation
These terms describe the rotation of the π-molecular orbitals involved in the resulting / opening bond. In the case of a conrotatory rotation, the direction of rotation of the two orbitals is identical, in the case of a disrotatory rotation it is opposite.
Examples
- Sigmatropic rearrangements
- Cope rearrangement , Claisen rearrangement (= Oxa-Cope rearrangement)
- Electrocyclic reactions
- Valence isomerization of benzene ( Prisman , Dewar-Benzol , Benzvalen ), Bullvalen
-
Cycloaddition
- thermal
- Diels-Alder reaction
- Dipolar cycloaddition
- Cheletropic reaction
- [2 + 2] cycloaddition ( antarafacial )
- photochemical
- [2 + 2] cycloaddition ( suprafacial ) ( Paternò-Büchi reaction )
- thermal
Individual evidence
- ↑ Robert B. Woodward, Roald Hoffmann: The preservation of orbital symmetry. Verlag Chemie, Weinheim 1970, ISBN 3-527-25323-8 .
- ↑ The Nobel Prize in Chemistry 1981 "for their theories, developed independently, concerning the course of chemical reactions"
- ^ Entry on Woodward-Hoffmann rules. In: Römpp Online . Georg Thieme Verlag, accessed on October 15, 2012.
literature
- Robert B. Woodward, Roald Hoffmann: Stereochemistry of Electrocyclic Reactions. In: Journal of the American Chemical Society. Volume 87, 1965, pp. 395-397.
- Robert B. Woodward, Roald Hoffmann: The Conservation of Orbital Symmetry. In: Angew. Chem. Int. Ed. Volume 8, 1969, pp. 781-853.
- Robert B. Woodward, Roald Hoffmann: The preservation of the orbital symmetry. Verlag Chemie, 1970. (English edition Academic Press 1970, The conservation of orbital symmetry)
- P. Wieland, H. Kaufmann: The Woodward-Hoffmann rules introduction and handling. Birkhäuser Verlag, Basel / Stuttgart 1972, ISBN 3-7643-0576-2 .
- Ian Fleming : Frontier Orbitals and Reactions of Organic Compounds. 1. corrected reprint. VCH Wiley, Weinheim et al. 1988, ISBN 3-527-25792-6 .