Benzvalen
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
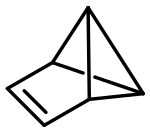
|
||||||||||
| General | ||||||||||
| Surname | Benzvalen | |||||||||
| Molecular formula | C 6 H 6 | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 78.11 g mol −1 | |||||||||
| Physical state |
liquid |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Benzvalene is an unstable organic compound and is isomeric to benzene . The structure is exactly the structure of C 6 H 6 predicted by Wade's rules , which has 6 × 3 = 18 framework electrons available, of which 6 × 2 = 12 are required for the binding orbitals. This leaves 6 x 1 = 6 electrons left what a arachno structure of the corresponding deltahedron with 8 corners ( trigondodecahedron equivalent), in which two adjacent corners were removed.
It was first synthesized in 1971 by Thomas J. Katz . The synthesis was carried out by reacting cyclopentadiene with methyl lithium in dimethyl ether and then with dichloromethane and methyl lithium at −45 ° C. The resulting solution has a putrid odor. Due to the high steric tension of the compound, it converts to benzene within a short time and tends to decompose explosively.
Benzvalene polymerizes through a ring-opening metathetic polymerization to polybenzvalene, which is being investigated as an intermediate in the production of polyethine .
Benzvalene can be converted into oxetanes by photochemical conversion .
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Manfred Christl: "Benzvalene - Properties and Synthetic Potential", in: Angewandte Chemie International Edition in English , 1981 , 20 (6-7), pp. 529-546; doi : 10.1002 / anie.198105291 .
- ↑ Thomas A. Albright, Jeremy K. Burdett, Myung-Hwan Whangbo: Orbital interactions in chemistry . Second ed. Wiley, Hoboken, New Jersey 2013, ISBN 978-0-471-08039-8 .
- ↑ Thomas J. Katz, E. Jang Wang, Nancy Acton: "Benzvalene synthesis", in: J. Am. Chem. Soc. , 1971 , 93 (15), pp. 3782-3783; doi : 10.1021 / ja00744a045 .
- ↑ Thomas J. Katz, Ronald J. Roth, Nancy Acton, Eileen Jang Carnahan: "Synthesis of Benzvalene", in: J. Org. Chem. , 1999 , 64 (20), pp. 7663-7664; doi : 10.1021 / jo990883g .
- ↑ a b Lawrence T. Scott, Maitland Jones: “Rearrangements and interconversions of compounds of the formula (CH) n”, in: Chem. Rev. , 1972 , 72 (2), pp. 181-202; doi : 10.1021 / cr60276a004 .
- ↑ Manfred Christl, Max Braun: “Photocycloadditions des Benzvalens”, in: Angewandte Chemie , 1989 , 101 (5), pp. 636–638; doi : 10.1002 / anie.19891010524 .