Oxetanes
| Oxetanes |
|---|
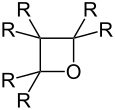 Oxetane (general formula) |
 Oxetane |
Oxetanes are heterocyclic organic chemical substances that contain a four-membered ring consisting of one oxygen atom and three carbon atoms. The unsubstituted parent of this group of substances is oxetane with the empirical formula C 3 H 6 O.
α-Oxo derivatives of the oxetanes are the cyclic β-lactones, which form the substance group of the oxetan-2-ones .
Manufacturing
Alcohols with a leaving group (e.g. halogen, arylsulfonyl, mesityl) in the γ position can, under the action of a base, form an alcoholate that cyclizes to the corresponding oxetane:
Paterno-Büchi reaction
An aldehyde or ketone can react with an alkene under the influence of light in the sense of a [2 + 2] cycloaddition , with an oxetane being formed:
Reactivity
Lewis acids such as boron trifluoride (BF 3 ) can be added to a non-bonding electron pair on the O atom of the oxetane. Cyclooligomerization then takes place in dichloromethane as solvent. The main product is cyclotrimer 1 :
Linear polyethers are formed under different reaction conditions, especially in the presence of water.
Individual evidence
- ↑ a b c d Theophil Eicher , Siegfried Hauptmann , Andreas Speicher: The Chemistry of Heterocycles , Wiley-VCH, 2012, ISBN 978-3-527-32747-8 , pp. 45-48.


