Phoron
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Phoron | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 9 H 14 O | |||||||||||||||
| Brief description |
Yellow liquid or yellow-green prisms |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 138.20 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
0.88 g cm −3 |
|||||||||||||||
| Melting point |
28 ° C |
|||||||||||||||
| boiling point |
197 ° C |
|||||||||||||||
| solubility |
poorly soluble in water |
|||||||||||||||
| Refractive index |
1.497 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
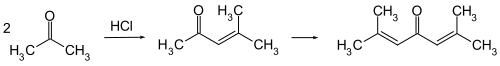
Phoron is the common name of a product of the condensation of acetone . The systematic name is 2,6-dimethylhepta-2,5-dien-4-one. The English common name ( phorones ) to a portmanteau of cam phor and acet one be.
The compound is one of the oldest representatives of the compound class of the so-called α, β-unsaturated ketones , which were later called conjugated enones . Phoron is yellow in color and therefore historically played a role in the theories of constitution and color.
Presentation and extraction
It was obtained in 1849 by Gerhardt and Liès-Bodart by distilling camphoric lime.
Phoron is formed when acetone reacts with dry hydrogen chloride , the main product being mesityl oxide . This condenses with acetone to form the phoron (double condensation of the acetone).
The reaction is a simple aldol condensation to form mesityl oxide and a double aldol condensation to form the phoron.
properties
Phoron forms yellowish green crystals with a geranium-like odor. It has a low melting point of 28 ° C. The compound boils at 197 ° C. under normal pressure. According to Antoine, the vapor pressure function results from log 10 (P) = A− (B / (T + C)) (P in bar, T in K) with A = 5.1950, B = 2259.288 and C = −35.106 in the temperature range from 315 to 470 K. Phoron is readily soluble in ethanol and ether and only slightly soluble in water.
use
The compound is used as a solvent for nitrocellulose and paints. Phoron can be used in food analysis to determine thiols. It is a versatile starting material in organic synthesis, e.g. B. for the production of 2,2,6,6-tetramethyl-4-piperidone , of 2,2,6,6-tetramethylpiperidine or the stable free radical TEMPO ( 2,2,6,6-tetramethylpiperidinyloxyl ). It is also an intermediate in Michael additions or in heterocycle syntheses.
Individual evidence
- ↑ a b c d e f g h Entry for CAS no. 504-20-1 in the GESTIS substance database of the IFA , accessed on October 7, 2012(JavaScript required) .
- ^ Adolf von Baeyer : Synthesis of Neurin. In: Annals of Chemistry and Pharmacy. 140, 1866, pp. 306-313, doi : 10.1002 / jlac.18661400308 . ( limited preview in Google Book search)
- ↑ Data sheet 2,6-Dimethyl-2,5-heptadien-4-one, 95% from Sigma-Aldrich , accessed on October 7, 2012 ( PDF ).
- ↑ a b L. F. Fieser and M. Fieser, Textbook of Organic Chemistry, translated and edited by HR Hensel, p. 222, Verlag Chemie, Weinheim, 1954.
- ↑ August Kekulé: Textbook of organic chemistry ... Ferdinand Enke, 1866, p. 463 ( limited preview in Google Book search).
- ↑ a b c d e f Entry on Phoron. In: Römpp Online . Georg Thieme Verlag, accessed on June 14, 2014.
- ↑ CRC Handbook of Data on Organic Compounds, 2nd Edition, Weast, RC and Grasselli, JG, ed (s)., CRC Press, Inc., Boca Raton, FL, 1989, 1.
- ↑ Stull, DR: Vapor Pressure of Pure Substances Organic Compounds in Ind. Eng. Chem. 39 (1947) 517-540, doi : 10.1021 / ie50448a022 .
literature
- Beyer-Walter: Textbook of Organic Chemistry , 21st edition, p. 223, Hirzel, Stuttgart, 1988.