Enones
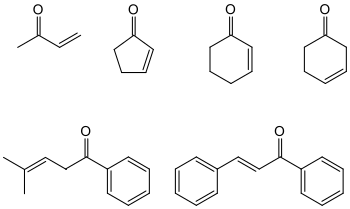
In organic chemistry, enones are ketones which, in addition to the C = O double bond (for which the suffix –on stands in the systematic substance name ), also contain a C = C double bond (suffix –en ). In short, the group of substances of this type is called en-ones . The carbon structure of the enones can be ring-shaped (cyclic) or acyclic; combinations of these skeletons are also possible.
An older term is " unsaturated ketones". According to the position (see persistence ) of the C = C double bond, a distinction is made between α, β-unsaturated ketones and β, γ-unsaturated, γ, δ-unsaturated etc. ketones, where α stands for a C atom adjacent to the carbon atom of the carbonyl group .
In the α, β-unsaturated ketones the two double bonds are " conjugated ". Often, α, β-unsaturated ketones are thermodynamically more stable than their double bond isomers. Therefore, the term “enone” is often restricted to the former.
nomenclature
However, Greek letters are also used in organic chemistry to describe other structures, e.g. B. in stereochemistry , or for naming crystal modifications. The position information with Greek letters is anchored in the history of organic chemistry and therefore can hardly be "eradicated". But they should be avoided in the nomenclature of individual compounds if possible.
With acyclic ketones, the longest chain of carbon atoms is numbered as with the alkanes . This gives the carbonyl group its position numbering and the suffix -on . The position of the C CC double bond is then entered, as with the numbering of the alkenes .
In cyclic ketones, the C = O group has the highest rank and is therefore assigned the number 1. The numbers ( locants ) of the C = C double bond should be as low as possible (starting from the O = C atom, one can clockwise or counterclockwise -Counting clockwise).
Many enones contain bicyclic or polycyclic carbon skeletons, e.g. B. steroids . In these cases the special numbering of these rings determines the position of the "- on ".
Many enones are traditionally named by their common names , especially natural substances. An instructive example is carvone : 2-methyl-5-isopropenyl-2-cyclohexen-1-one, more precisely: 2-methyl-5- (1-methylethenyl) -cyclohex-2-en-1-one.
Special properties of α, β-unsaturated ketones
While C = C and C = O double bonds generally do not influence each other in non-conjugated enones, an electronic interaction occurs between the two functional groups in enones, which is described by the term conjugation . The prerequisite is that the four atoms of the enone system (C = CC = O) lie in one plane or deviate only slightly from it. In other words, the enone system should be in as planar a conformation as possible .
The delocalization of the π electrons can be represented by the following mesomeric boundary structures:
The enone system is isoelectronic with the 1,3-diene system (prototype 1,3-butadiene); however, the polar boundary structure (right) becomes more important when the terminal carbon atom in the hydrocarbon is exchanged for an oxygen atom. Because oxygen has a higher electronegativity than carbon and "therefore attracts the electrons more strongly" (to put it casually). From this perspective it is also understandable that - compared to “normal” ketones - the O = C bond is longer, and the formal single bond = CC = shorter.
A second possibility to describe the enone function is offered by the molecular orbital theory , whereby the simple Hückel approximation shows decisive trends. The π molecular orbitals (π MOs) are polarized.
The special bonding conditions in the enones cause, among other things, characteristic changes in the IR spectra and UV spectra in comparison with simple ketones.
Reactivity of α, β-unsaturated ketones
Enones are bifunctional compounds; therefore, in the case of non-conjugated enones, the reactions characteristic of the O = C and C = C functions (alkene and ketone) are possible. As a rule, reactions with electrophilic agents take place at the C = C double bond, while nucleophiles attack the carbon atom of the carbonyl group.
In the case of the α, β-unsaturated ketones, however, the π-electron system is delocalized. With regard to its reactivity, the enone function must therefore be viewed as a unit. This changes the reactivity of these enones compared to "normal" ketones. Although the addition of nucleophiles to the carbon atom of the carbonyl group is often observed, but another type of reaction is the association of a nucleophile with the β-carbon atom of the enone system, the so-called 'conjugate addition' '(engl. Conjugate addition ). This includes the Michael addition and many reactions with organometallic compounds. If one looks at the polar boundary structure formulas (see above), this becomes plausible.
However, the models of molecular orbital theory have proven to be more suitable for understanding reactivity. In the context of the theory of frontier orbitals (HOMO-LUMO interactions), conjugated enones are characterized by LUMOs, which are relatively low on the energy scale. In reactions with nucleophilic agents - apart from Coulomb forces - the interaction of the enone LUMO with the HOMO of a nucleophile is important as a first approximation. The MO coefficients at the carbonyl carbon atom and the β carbon atom differ in size; soft nucleophiles therefore have a tendency to attack the latter. This also applies to many radicals.
Syntheses of α, β-unsaturated ketones
Numerous methods have been developed for the preparation of substances of this class of compounds, but only a selection can be discussed in this context. For practical application it is crucial that the starting materials , the starting materials, are easily accessible and as cheap as possible.
All ketones can be viewed as products of the dehydrogenation (oxidation) of secondary alcohols. If the corresponding unsaturated alcohols are available, they can be oxidized. Compared to the saturated alcohols, it takes place under "milder" conditions, e.g. B. with manganese dioxide . One example is the synthesis of 2-cyclopentenone.
-

From cyclopentadiene, 2-cyclopentenol is formed in a 1,4-addition of water. This is oxidized to the ketone by aqueous chromic acid.
In principle, saturated ketones can also be dehydrogenated, but selective dehydrogenation in the α, β position is difficult. A “classic” detour is the halogenation of saturated ketones in the α position; Hydrogen halide can usually be split off from the α-haloketones formed by bases (α, β-elimination). Bromine is usually chosen as the halogen. In this way dienones could be obtained from some α, α'-dibromoketones, such as 4,4-dimethyl-2,5-cyclohexanone.
-

4,4-Dimethyl-2,5-cyclohexadienone from 2,6-dibromo-4,4-dimethylcyclohexanone. Calcium carbonate in dimethylformamide (DMF) is used as the base.
The oldest synthesis principle is the condensation of aldehydes or ketones with another ketone (" aldol condensation "). So were benzylidene , dibenzylideneacetone , benzylideneacetophenone or Mesityloxide won for the first time. The semi-trivial names of the first three examples still indicate the origin of these compounds today.
Intramolecular variants of this reaction are also known: some dicarbonyl compounds yield cycloalkenones. For example, undecane-2,5-dione can be cyclized to dihydrojasmone , an important fragrance.
-

Cyclization of undecane-2,5-dione to dihydrojasmone. The reaction is carried out in boiling ethanol with 2 percent aqueous sodium hydroxide solution as a catalyst.
Individual evidence
- ↑ E. Heilbronner, H. Bock: The HMO model and its application. Vol. 2, Verlag Chemie, Weinheim 1970, pp. 165 and 170.
- ↑ Ian Fleming: Frontier Orgitals and Organic Chemical Reactions. Wiley, London et al. a. 1976, pp. 70, 163; German edition: Ian Fleming: Frontier orbitals and reactions of organic compounds. translated by Henning Hopf . VCH, Weinheim / Basel / Cambridge 1990, ISBN 3-527-25792-6 .
- ^ FG Bordwell, KM Wellman: In: J. Org. Chem. 28, (1963), p. 2544.
- ↑ Heinz Hunsdiecker: About the behavior of γ-diketones, 1st communication . In: Reports of the German Chemical Society . tape 75 , no. 5 , May 6, 1942, pp. 447-454 , doi : 10.1002 / cber.19420750502 .
literature
- Saul Patai (Ed.): The chemistry of enones (The chemistry of functional groups). Wiley, Chichester, et al. a. 1989.
- Part 1: ISBN 0-471-91563-7 .
- Part 2: ISBN 0-471-92289-7 .
