3-butene-2-one
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 3-butene-2-one | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 4 H 6 O | ||||||||||||||||||
| Brief description |
light-sensitive, volatile, colorless liquid with an unpleasant odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 70.09 g · mol -1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.83 g cm −3 |
||||||||||||||||||
| Melting point |
−7 ° C |
||||||||||||||||||
| boiling point |
81.4 ° C |
||||||||||||||||||
| Vapor pressure |
100 h Pa (20 ° C) |
||||||||||||||||||
| solubility |
miscible with water |
||||||||||||||||||
| Refractive index |
1.4081 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
3-buten-2-one (also methyl vinyl ketone ) is the simplest unsaturated ketone , a pungent-smelling liquid that polymerizes easily .
Extraction and presentation
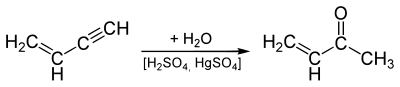
The compound can be made by hydrating vinyl acetylene ( butenine ) in the presence of sulfuric acid and mercury (II) sulfate :
properties
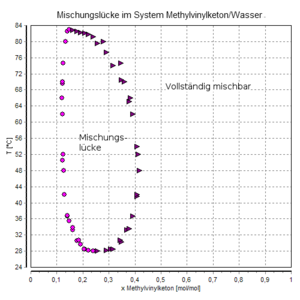
3-buten-2-one is a colorless, light-sensitive liquid that boils at 81 ° C under normal pressure . The compound is completely miscible with water below 28 ° C. Above this temperature, in the range exists between about 10 and 40 mole percent of a miscibility gap .
use
3-buten-2-one is used in the manufacture of gas-impermeable plastics and as an intermediate in the manufacture of pharmaceuticals , fungicides and other chemical compounds (e.g. 2-ethoxy-buta-1,3-diene or 1-phenyl-pentane-1 , 4-dione ) is used. It is implemented, for example, by a Diels-Alder reaction or Robinson annulation .
safety instructions
3-buten-2-one tends to form explosive peroxides and to spontaneous polymerization, which can be triggered by sunlight. The vapors of 3-butene-2-one form explosive mixtures with air. The compound has a flash point of −7 ° C. The explosion range is between 2.1 vol.% As the lower explosion limit (LEL) and 15.6 vol.% As the upper explosion limit (UEL). The ignition temperature is 491 ° C. The substance therefore falls into temperature class T1.
Individual evidence
- ↑ a b c d e f g h i j k Entry on butenone in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ a b H. Beyer u. W. Walter: Textbook of Organic Chemistry . 20th ed., Hirzel, Stuttgart, 1984. p. 211.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-74.
- ↑ Dortmund database .