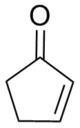
2-cyclopenten-1-one
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 2-cyclopenten-1-one | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 5 H 6 O | |||||||||||||||
| Brief description |
light yellow liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 82.10 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.98 g cm −3 (25 ° C) |
|||||||||||||||
| boiling point |
64-65 ° C (19 mmHg) |
|||||||||||||||
| solubility |
almost insoluble in water |
|||||||||||||||
| Refractive index |
1.481 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
2-Cyclopenten-1-one is a chemical compound from the group of the enones and isomeric to 3-Cyclopenten-1-one .
Occurrence
2-Cyclopenten-1-one occurs naturally as the basic structure in a number of chemical compounds such as jasmine .
Extraction and presentation
In general, cyclopentenones can be synthesized in a number of ways. The most widespread industrial route is the dehydrohalogenation of 2-bromocyclopentanone with lithium carbonate and the Claisen condensation - decarboxylation - isomerization of unsaturated diesters reaction cascade .
Synthesis from cyclopentadiene is also possible , with 1,4-addition with water forming 2-cyclopentenol , which is oxidized to the ketone by aqueous chromic acid .
There are other routes available for the synthesis of substituted cyclopentenones: Nazarov cyclization reaction of divinyl ketones, Saegusa – Ito oxidation of cyclopentanones , ring-closing metathesis of the associated dienes , oxidation of the associated cyclic allyl alcohols and the Pauson-Khand reaction of alkenes , Alkynes and carbon monoxide .
properties
2-Cyclopenten-1-one is a light yellow liquid that is insoluble in water.
use
2-Cyclopenten-1-one can be used to make other chemical compounds (such as cubane ).
Individual evidence
- ↑ a b c d e f g h Data sheet 2-Cyclopenten-1-one, 98% from Sigma-Aldrich , accessed on January 6, 2013 ( PDF ).
- ↑ a b Data sheet 2-Cyclopenten-1-one (PDF) from Fisher Scientific , accessed on February 13, 2014.
- ↑ US EP1418166, Daisuke, Fukushima & Hirata Norihiko, "Process for producing 2-bromocyclopentanone", published May 12, 2004
- ↑ US EP1422212, Liang, Shelue; Andrea Haunert & Sylvia Huber-Dirr et al., "Process for preparing cyclopentenone", published November 25, 2004.
- ↑ Organic Reactions Portal: Cyclopentenone synthesis


