Cuban
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||
| General | ||||||||||
| Surname | Cuban | |||||||||
| other names |
Pentacyclo [4.2.0.0 2.5 .0 3.8 .0 4.7 ] octane |
|||||||||
| Molecular formula | C 8 H 8 | |||||||||
| Brief description |
shimmering, colorless rhombuses |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 104.14 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| density |
1.29 g cm −3 |
|||||||||
| Melting point |
130-131 ° C |
|||||||||
| boiling point |
200 ° C (decomposition) |
|||||||||
| Vapor pressure |
146.7 Pa (25 ° C) |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Cubane is a synthetic hydrocarbon , the molecule of which consists of eight carbon atoms arranged in the shape of a cube, each with an associated hydrogen atom . The carbon structure encloses a cavity, so cubane belongs to the class of cage compounds , more precisely to the platonic hydrocarbons . Until its first synthesis in 1964, it was initially only considered to exist in theory and, because of the unusually acute 90-degree bond angle of the carbon atoms, was considered unstable. Due to this 90-degree angle, cubane stores a lot of energy in these bonds, so it can be used as a base for high-energy propellants or explosives (see also tetranitrocubane , octanitrocubane ).
history
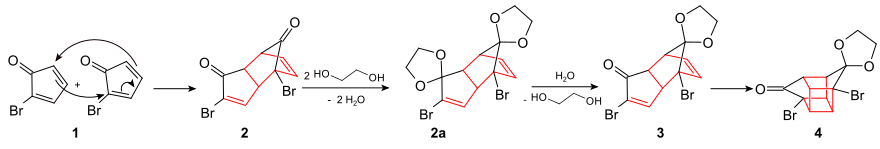
The presentation of Cubane in a thirteen-step synthesis by Philip Eaton was first published in 1964. The original synthesis starts from 2-cyclopenten-1-one ( 1 ), which is first monobrominated to ( 2 ) in a Wohl-Ziegler reaction with N- bromo succinimide. The tribromo compound ( 3 ) is obtained by further bromination with elemental bromine , from which the actual starting compound of the cubane synthesis 2-bromocyclopentadienone ( 4 ) is obtained by dehydrohalogenation :
This starting compound (2-bromocyclopentadienone) is dimerized to ( 2 ) in a first step via a Diels-Alder reaction . Both carbonyl groups in ( 2 ) are protected with ethylene glycol ( 2a ). Subsequently, that is endo -ständige acetal group selectively to form the compound ( 3 ) is hydrolyzed . It includes a intramolecular photochemical [2 + 2] - cycloaddition bromo ketone ( 4 ) to:
The bromoketone obtained in this way is rearranged via a Faworski rearrangement by ring contraction to form the carboxylic acid ( 5 ). The free carboxylic acid ( 5 ) is in a peroxycarboxylic acid ester ( 6 transferred) and then thermally ( 7 ) decarboxylated :
Finally, the remaining acetal group is also hydrolyzed, the bromoketone ( 8 ) rearranged in a second Faworski reaction and the carboxylic acid ( 9 ) formed is converted into the corresponding peroxycarboxylic acid ester ( 10 ). The target molecule cubane ( 11 ) is obtained after a thermal decarboxylation of ( 10 ):
synthesis
The cubane structure can be built up in a simpler five-stage synthesis initially to give cubane-1,4-dicarboxylic acid. The starting compound of the synthesis is cyclopentanone , which is converted to the cyclic ketal in the first step using ethylene glycol . Bromination produces a triple brominated cyclopentanone ketal which, after dehydrohalogenation and Diels-Alder reaction, is converted into a polycyclic Diels-Alder intermediate. An intramolecular photochemical [2 + 2] cycloaddition leads to a partial cubane structure, which is completed by a reaction under reflux in the presence of sodium hydroxide solution. The overall yield is about 25% over all reaction stages. The synthesis has been carried out down to the kilogram scale.
Cubane-1,4-dicarboxylic acid is a basic compound for the synthesis of other substituted cubane compounds. The decarboxylation to cubane takes place in two steps via the tert-butyl perester. An almost quantitative synthesis is achieved through the photochemical degradation of a thiohydroxamic acid ester.
properties
Physical Properties
Cubane is a solid, crystalline substance at room temperature that occurs in two polymorphic crystal forms. At room temperature, the crystal form II is present, which at 121.9 ° C converts to the crystal form I in a first-order phase transition . This crystal form is in a plastic crystalline form. This means that the connection between the two phase transitions is in a mesomorphic state . The liquid phase is reached at 131.8 ° C. The vapor pressure function is given by August corresponding log 10 (P) = -A / T + B (P in Torr T in K) where A = 2200 and B = 8. The compound is having a standard enthalpy of formation of Δ f H solid = 542 kJ mol −1 or Δ f H gas = 622 kJ mol −1 strongly endothermic. The standard enthalpy of combustion Δ c H solid is −4833.27 kJ mol −1 .
At room temperature, cubane crystallizes in a trigonal crystal structure with the space group R3 with one molecule per unit cell. The bond lengths determined by electron diffraction are 157.27 ± 0.19 pm for the C – C bond, 111.8 ± 0.8 pm for the C – H bond and differ only slightly from those in cyclobutane at 155.1 pm for the C – C bond and 109 pm for the C – H bond.
Chemical properties
In spite of the highly tensioned ties, the connection is stable. A measurable decomposition is only observed at temperatures above 200 ° C. The activation energy for thermolysis is relatively high with a value of 180.5 kJ · mol −1 . Cubane is also relatively stable to light, air and water. Cubane can undergo intramolecular, metal-catalyzed bond rearrangements. In the presence of silver or palladium catalysts, the reaction leads to the cunean .
With rhodium catalysts, syn- tricyclooctadiene is initially formed, which can be thermally converted to cyclooctatetraene at 50–60 ° C.
literature
- PE Eaton: Cubanes: Starting Compounds for Chemistry in the Nineties and the Next Century . In: Angew. Chem. Band 104 , 1992, pp. 1447–1462 , doi : 10.1002 / anie.19921041105 .
Individual evidence
- ↑ a b Entry on Cuban. In: Römpp Online . Georg Thieme Verlag, accessed on December 28, 2014.
- ↑ Jai Prakash Agrawal: High Energy Materials: Propellants, Explosives and Pyrotechnics , Wiley-VCH Verlag GmbH & Co. KGaA 2010, ISBN 978-3-527-32610-5 , p. 138.
- ^ The Merck Index . An Encyclopaedia of Chemicals, Drugs and Biologicals. 14th edition, 2006, ISBN 978-0-911910-00-1 , p. 439.
- ↑ a b L. Hedberg, K. Hedberg, EP E Kenneth, N. Nodari, AG Robiette: Bond lengths and quadratic force field for cubane. In: J. Am. Chem. Soc. 113, 1991, pp. 1514-1517, doi: 10.1021 / ja00005a007 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b P. E. Eaton, TW Cole: Cubane. In: J. Am. Chem. Soc. 86 1964, pp. 3157-3158, doi: 10.1021 / ja01069a041 .
- ^ PE Eaton, TW Cole: The Cubane System. In: J. Am. Chem. Soc. 86, 1964, pp. 962-964, doi: 10.1021 / ja01059a072 .
- ^ NB Chapman, JM Key, KJ Toyne: The Preparation and Properties of Cage Polycyclic Systems - I. Pentacyclo [5.3.0.0 2.5 .0 3.9 .0 4.8 ] decane and pentacyclo [4.3.0.0 2.5 .0 3.8 .0 4.7 ] nonane derivatives. In: J. Org. Chem. 35, 1970, pp. 3860-3867. doi: 10.1021 / jo00836a062 .
- ↑ a b c d e f g P. E. Eaton: Cubanes: starting compounds for chemistry of the 1990s and the next century. In: Angew. Chem. , 104, 1992, pp. 1447-1462, doi: 10.1002 / ange.19921041105 .
- ↑ DHR Barton, D. Crich, WB Motherwell: New and improved methods for the radical decarboxylation of acids. In: J. Chem. Soc., Chem. Commun. 1983, pp. 939-941, doi: 10.1039 / C39830000939 .
- ↑ a b c d e M. A. White, RE Wasylishen, PE Eaton, Y. Xiong, K. Pramod, N. Nodari: Orientational Disorder in Solid Cubane: A Thermodynamic and 13 C NMR Study. In: J. Phys. Chem. , 96, No. 1, 1992, pp. 421-425, doi: 10.1021 / j100180a078 .
- ↑ a b B. D. Kybett, S. Carroll, P. Natalis, DW Bonnell, JL Margrave, JL Franklin: Thermodynamic Properties of Cubane. In: J. Am. Chem. Soc. , 88, 1966, pp. 626-626, doi: 10.1021 / ja00955a056 .
- ↑ EB Fleischer: X-Ray Structure Determination of Cubane. In: J. Am. Chem. Soc. , 86, 1964, pp. 3889-3890, doi: 10.1021 / ja01072a069 .
- ^ NL Allinger, PE Eaton: The geometries of pentaprismane and hexaprismane insights from molecular mechanics. In: Tetrahedron Lett. , 24, 1983, pp. 3697-3700, doi: 10.1016 / S0040-4039 (00) 94512-X .
- ^ NL Allinger: Conformational Analysis. 130. MM2. A Hydrocarbon Force Field Utilizing V 1 and V 2 Torsional Terms. In: J. Am. Chem. Soc. , 99, 1977, pp. 8127-8134, doi: 10.1021 / ja00467a001 .
- ↑ H.-D. Martin, T. Urbanek, P. Pföhler, R. Walsh: The pyrolysis of cubane; an example of a thermally induced hot molecule reaction. In: J. Chem. Soc., Chem. Commun. , 1985, pp. 964-965, doi: 10.1039 / C39850000964 .
- ^ PE Eaton, L. Cassar, J. Halpern: Silver (I) - and Palladium (II) -Catalyzed Isomerizations of Cubane. Synthesis and Characterization of Cuneane. In: J. Am. Chem. Soc. , 92, 1970, pp. 6366-6368, doi: 10.1021 / ja00724a061 .
- ↑ L. Cessar, PE Eaton, J. Halpern: Catalysis of Symmetry-Restricted Reactions by Transition Metal Compounds. The Valence Isomerization of Cubane. In: J. Am. Chem. Soc. , 92, 1970, pp. 3515-3518, doi: 10.1021 / ja00714a075 .