Faworski rearrangement
The Faworski rearrangement (often Favorski rearrangement written English rearrangement Favorski ) is a name reaction in organic synthetic chemistry , which after the Russian chemist Alexey Favorsky was named (1860-1945). The Faworski rearrangement in its simplest form describes the rearrangement of enolizable α-haloketones to carboxylic acids with the addition of hydroxides (strong bases). By varying the base used, carboxylic acid esters and carboxamides are also accessible.
The Faworski rearrangement should not be confused with the Faworski reaction .
history
The Faworski rearrangement of α-haloketones in a basic environment leads to carboxylic acids or carboxylic acid esters . Starting substances mostly chlorine or bromine ketones, rarely iodine ketones. Suitable bases are hydroxides , alcoholates and amines . Carboxamides can be formed with ammonia .
α-chlorocyclohexanone provides with potassium hydroxide solution with ring contraction the potassium salt of cyclopentanecarboxylic acid , which can be converted by neutralization to cyclopentane carboxylic acid:
Faworski recognized that treating some aliphatic dihaloketones of the RCH 2 CX 2 COCH 2 R 'type with dilute potassium hydroxide solution results in α, β-unsaturated carboxylic acids .
Reaction mechanism
The exact sequence of the rearrangements named after Faworski has long been a matter of dispute. Finally, it was recognized that at least two mechanisms are possible. So that today a distinction is made between Faworski rearrangement and quasi-Faworski rearrangement (English quasi-Favorskii rearrangement ).
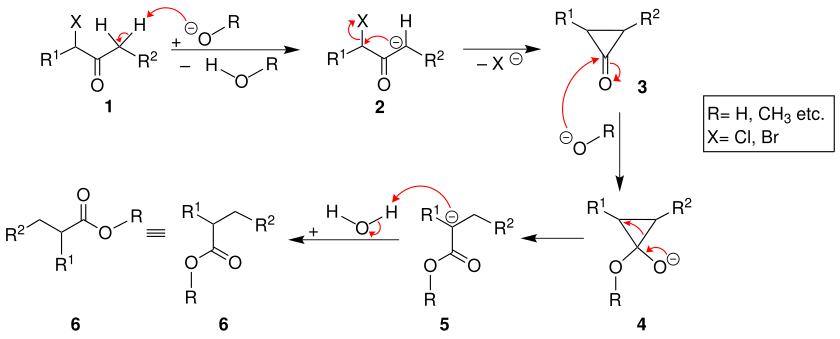
The mechanism of the Faworski rearrangement begins with the deprotonation of the α-haloketone 1 by the base used. The carbanion 2 , which is stabilized by keto-enol tautomerism , is formed, which, after the halide ion has been split off, passes into the cyclopropanone intermediate 3 . The positively polarized carbon atom of the carbonyl group is then attacked nucleophilically by the base used. The result is compound 4 , which is protonated by a water molecule after rearrangement. The desired connection is created 6 .
If 1 is reacted with potassium hydroxide or sodium hydroxide solution, a carboxylic acid is formed (R = H). If, on the other hand, 1 is reacted with an alcoholate, the reaction product 6 is a carboxylic acid ester 6 (R = organyl radical , such as, for example, an alkyl radical) and a carboxamide is obtained when using amines.
An example of the mechanism is the reaction of α-chlorocyclohexanone 7 . Here, too, an intermediate stage of the cyclopropanone structure 8 occurs, as Loftfield proved by labeling with 14 C.
Quasi-Faworski rearrangement
In the case of non-enolizable (or only extremely slowly enolizable) α-haloketones, a different mechanism takes place. This can be the case if there are no α-hydrogen atoms or if a double bond, which is decisive for the enol form, is difficult to form due to ring tension (see Bredt's rule ). This mechanism begins with the nucleophilic attack of the base used on the positively polarized carbon atom of the α-haloketone 9 (X = Cl, Br; R 1 to R 4 = H, alkyl). The halide is then split off and - depending on the base used - a carboxylic acid 11 (R = H) or a carboxylic acid ester 11 (R = alkyl) is obtained: In contrast to the regular Faworski rearrangement, no cyclopropanone intermediate is observed.
While this rearrangement is not known in the case of chlorocyclopentanone, 2-halocyclobutanone 12 yields the cyclopropanecarboxylic acid 14 with ring constriction , in addition to 2-hydroxycyclobutanone and represents an example of the mechanism described.
The French chemists provided evidence that, in this case, hydroxide attacks the carbonyl carbon atom and the neighboring CC bond is cleaved. This reaction mechanism is similar to the benzilic acid rearrangement and is therefore also known as the “semibenzilic acid rearrangement”.
use
In organic synthesis, Faworski rearrangements are used whenever alternative syntheses of carboxylic acid (esters) n are more laborious than those of α-haloketones.
The Faworski rearrangement is widely used in cyclic compounds, as an n-membered ring can usually be converted into the next smaller (n - 1) -membered ring (so-called ring narrowing).
A synthesis of the strained hydrocarbon cubane by Eaton, in which a quasi-Faworski rearrangement was a key step, attracted particular attention .
literature
- Christian M. Rojas: Molecular Rearrangements in Organic Synthesis . John Wiley & Sons, Inc., Hoboken, New Jersey, 2015, ISBN 978-3-540-30030-4 , doi : 10.1002 / 9781118939901.ch7 .
- Robert Jacquier: Rearrangement des cétones α-halogénées en acides sous l'influence des reactifs alcalins (Reaction de Faworsky). In: Bull. Soc. Chim. France. 1950, pp. 35-45.
- Andrew S. Kende: The Favorskiĭ Rearrangement of Haloketones . In: Organic Reactions . No. 11 , 1960, pp. 261-316 , doi : 10.1002 / 0471264180.or011.04 .
- Jean M. Conia, Jacques R. Salaun: Cyclobutane ring contractions not involving carbonium ions . In: Accounts of Chemical Research . tape 5 , no. 1 , January 1, 1972, p. 33-40 , doi : 10.1021 / ar50049a005 .
- J. Bülle, A. Hüttermann: The basic knowledge of organic chemistry. Wiley-VCH, 2000.
Web links
Individual evidence
- ↑ a b A. E. Faworskii: J. Russ. Phys. Chem. Band 26 , 1894, pp. 559 . German version: A. Faworski: Journal for practical chemistry . tape 51 , 1895, p. 533 .
- ^ Jie Jack Li: Name Reactions - A Collection of Detailed Reaction Mechanisms . Ed .: Springer Berlin Heidelberg. 2006, ISBN 978-3-540-30030-4 , pp. 220–221 , doi : 10.1007 / 3-540-30031-7_99 ( springer.com [accessed November 27, 2016]).
- ↑ A. Faworski, W. Boshowski: Isomeric Transformations of Cyclic α-Monocbloroketones. In: J. Russ. Phys. Chem. 46, 1914, pp. 1097-1102. German version: About isomeric transformations of cyclic α-monochloro ketones. In: Chemisches Zentralblatt . 1915, I, p. 984.
- ↑ a b c Michael Harmata: Molecular Rearrangements in Organic Synthesis . John Wiley & Sons, Inc, 2015, ISBN 978-1-118-93990-1 , pp. 183-226 , doi : 10.1002 / 9781118939901.ch7 .
- ↑ a b Robert Berner Loftfield: The alkaline rearrangement of α-Haloketones. II. The Mechanism of the Faworskii Reaction . In: Journal of the American Chemical Society . tape 73 , no. 10 , October 1, 1951, p. 4707-4714 , doi : 10.1021 / ja01154a066 .
- ^ A b Z. Wang: Comprehensive Organic Name Reactions and Reagents. 3 volumes. Volume 1, Wiley, 2009, ISBN 978-0-471-70450-8 , p. 1025.
- ^ Edward E. Smissman, Gilbert Hite: The Quasi-Favorskii Rearrangement. I. The Preparation of Demerol and β-Pethidines . In: Journal of the American Chemical Society . tape 81 , no. 5 , March 1, 1959, p. 1201–1203 , doi : 10.1021 / ja01514a047 .
- ^ T. Laue, A. Plagens: Name and keyword reactions of organic chemistry. 5th edition, Teubner Studienbücher Chemie, 2006, p. 122.
- ↑ JM Conia, J. Salaün: Mecanisme de la transposition de Favorski de la bromo-2 cyclobutanone . In: Tetrahedron Letters . tape 4 , no. 18 , 1963, p. 1175-1177 , doi : 10.1016 / S0040-4039 (01) 90798-1 .
- ↑ Jean M. Conia, Jacques R. Salaun: Cyclobutanes ring contractions not Involving carbonium ions . In: Accounts of Chemical Research . tape 5 , no. 1 , January 1, 1972, p. 33-40 , doi : 10.1021 / ar50049a005 .
- ↑ Bianca Tchoubar, Otto Sackur: Déshalogénation alcaline de la chloro-I cyclohexylméthylcétone et de la chloro-1 cyclohexylphénytone. Transposition en acidic cyclohexylformiques α substités . In: Comptes rendus hebdomadaires des séances de l'Académie des sciences . tape 208 , 1939, pp. 1020-1022 ( digitized on Gallica ).
- ^ Philip E. Eaton, Thomas W. Cole: The Cubane System . In: Journal of the American Chemical Society . tape 86 , no. 5 , March 1, 1964, pp. 962-964 , doi : 10.1021 / ja01059a072 .
- ^ Philip E. Eaton, Thomas W. Cole: Cubane . In: Journal of the American Chemical Society . tape 86 , no. 15 , August 1, 1964, p. 3157-3158 , doi : 10.1021 / ja01069a041 .