Bredt's rule
The Bredt's rule is an empirical rule in the organic chemistry . It was first formulated by the German chemist Julius Bredt in 1924 . It states that in small bicyclic hydrocarbons, due to the ring tension, no double bond can be on a bridgehead atom .
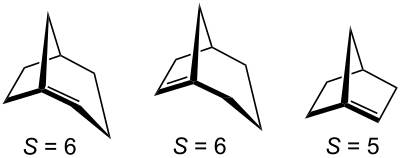
In the meantime, Bredt's rule has been specified. According to this, only those bicyclo [xyz] alk-1-enes are stable in which the sum S of the bridge members fulfills the following requirement: S = x + y + z ≥ 7.
However, some unstable bicyclic hydrocarbons with S = 5 or 6 could be detected indirectly by subsequent reactions.
One can imagine that a double bond at the bridgehead would correspond to a trans double bond , which hardly occurs due to the resulting ring tension for ring systems with fewer than eight members. As a result of the geometry, the overlap of the p orbitals of the sp 2 - hybridized carbon atoms involved in the bond would be too small to ensure a stable bond. Analogous to trans double bonds in cyclic hydrocarbons , double bonds at the bridgehead of bicyclic compounds only appear from a size of eight ring atoms.
There are also some compounds that do not follow Bredt's rule. These are also known as anti-Bredt compounds. An example is the 1 (9) homocube synthesized by Philip E. Eaton and Karl L. Hoffmann , whereby the term olefin can only be used to a limited extent according to theoretical studies - due to the strong twisting of the p orbitals, which make the π- Realize bond. The 1 (9) homocube is currently considered to be the olefin with the strongest twist.
Individual evidence
- ↑ J. Bredt: About steric hindrance in bridge rings (Bredt's rule) and about the meso - trans position in condensed ring systems of hexamethylene , in: Liebigs Ann. 1924 , 437 , 1-13; doi : 10.1002 / jlac.19244370102 .
- ^ A b Siegfried Hauptmann : Organic Chemistry , 2nd edition, VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig, 1985, p. 227, ISBN 3-342-00280-8 .
- ^ Ulrich Lüning: Organic reactions , 2nd edition, Elsevier GmbH, Munich, 2007, p. 59, ISBN 978-3-8274-1834-0 .
- ↑ PE Eaton, KL Hoffmann: 9-Phenyl-1 (9) -homocubene, probably the most twisted olefin yet known, and the carbene 1-phenyl-9-homocubylidene, its rearrangement product , in: J. Am. Chem. Soc. 1987 , 109 , 5285-5286; doi : 10.1021 / ja00251a047 .
- ↑ MC Holthausen, W. Koch: 1 (9) -homocubes and 9-homocubylids: theoretical investigation of structures, energies and rearrangement reactions , in: Angew. Chem. 1994 , 106 , 682-684; doi : 10.1002 / anie.19941060614 .
- ↑ PE Eaton: Cubane: Starting Compounds for the Chemistry of the Nineties and the Next Century , in: Angew. Chem. 1992 , 104 , 1447-1462; doi : 10.1002 / anie.19921041105 .