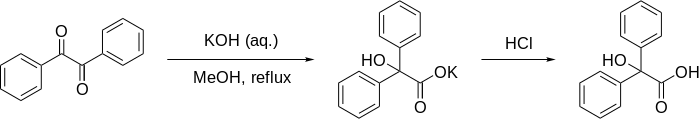
Benzilic acid rearrangement
The benzilic acid rearrangement is a rearrangement reaction of 1,2-diketones to α - hydroxycarboxylic acids in the presence of strong bases such as potassium hydroxide . It is closely related to the Cannizzaro reaction and the benzidine rearrangement and was first described in 1838 by Justus Liebig (1803–1873).
reaction
The benzylic acid as a name of the reaction caused by a benzilic acid rearrangement of benzil (dibenzoyl).
This diketone reaction is similar to other rearrangements: the corresponding ketoaldehyde (an alkyl or aryl group is replaced by hydrogen) rearranges in a Cannizzaro reaction, the 1,2-diol reacts according to the pinacol rearrangement .
Reaction mechanism
The following reaction is typical of 1,2 rearrangements. Usually at this rearrangement carbocations involved, but here come carbanions to reaction. This reaction mechanism, which has been known for a long time, has been confirmed by in silico investigations and is detailed below. A hydroxide anion attacks one of the keto groups of 1 in a nucleophilic addition and forms a hydroxide anion 2 . The next step is a rotation around the central CC-axis in order to obtain the conformer 3 . The radicals R are cis to one another. This is necessary so that the migrating residue can attack the second carbonyl group in a concerted reaction and can reverse the resulting hydroxyl group to form the first carbonyl group. This step is similar to a nucleophilic acyl substitution.
The carboxylic acid in intermediate step 4 is less basic than the hydroxide anion. The proton therefore migrates to intermediate stage 5 , which in turn can be protonated by acid and the end product α - hydroxycarboxylic acid 6 is formed .
In the case of cyclic 1,2-diketones, ring strain occurs .
The benzylic acid as a name of the reaction caused by a benzilic acid rearrangement of benzil (dibenzoyl).
variants
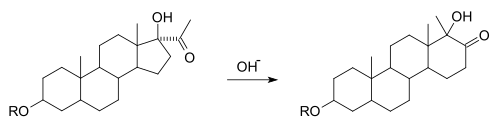
A variant of rearrangement occurs in the synthesis of various steroids . In the D-homo rearrangement of steroids , a cyclopentane ring (" D-ring ") is expanded under the action of a base to form a cyclohexane ring, ie it is converted into the next higher homologue .
literature
- Donald A. Ballard and William M. Dehn: Benzilic acid In: Organic Syntheses . 1, 1921, p. 29, doi : 10.15227 / orgsyn.001.0029 ; Coll. Vol. 1, 1941, p. 89 ( PDF ).
Web links
- Laboratory instruction I ( Memento from June 13, 2010 in the Internet Archive ) (PDF file; 472 kB)
- Laboratory instruction II
Individual evidence
- ↑ Liebig, J .: About Laurent's theory of organic compounds . In: Annals of Chemistry . 25, 1838, pp. 1-31. doi : 10.1002 / jlac.18380250102 .
- ↑ Shinichi Yamabe, Noriko Tsuchida, and Shoko Yamazaki: A FMO-Controlled Reaction Path in the Benzil-Benzilic Acid Rearrangement . In: J. Org. Chem. . 71, No. 5, 2006, pp. 1777-1783. doi : 10.1021 / jo051862r .
- ↑ L. Ruzicka , HF Meldahl: About steroids and sex hormones. 48th communication. The conversion of 17-ethynyl-androstene derivatives into pregnenone derivatives. Production of the 17-oxy-progesterone Helvetica Chimica Acta 21 ( 1938 ) 1760–1770, doi : 10.1002 / hlca.193802101214 .
- ↑ L. Ruzicka, HF Meldahl: About steroids and sex hormones. (Part 51). The production of neo-pregnenolone from 5-3, 17-dioxypregnenone- (20) Helvetica Chimica Acta 22 ( 1939 ) 421-424, doi : 10.1002 / hlca.19390220155 .