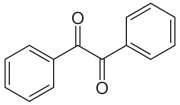
Benzil
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Benzil | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 14 H 10 O 2 | ||||||||||||||||||
| Brief description |
yellow crystals with a characteristic odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 210.23 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.23 g cm −3 (25 ° C) |
||||||||||||||||||
| Melting point |
95 ° C |
||||||||||||||||||
| boiling point |
Decomposition from 346 ° C |
||||||||||||||||||
| solubility |
poor in water (> 0.5 g l −1 at 20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Benzil is a diketone and has the formula C 6 H 5 –CO – CO – C 6 H 5 .
Extraction and presentation
Benzil can be obtained by a benzoin addition of benzaldehyde and subsequent oxidation of the benzoin with copper sulfate . Another production variant is the oxidation of diphenylethine using peroxomonophosphoric acid .
properties
Physical Properties
Benzil is a crystalline solid that can appear in two polymorphic crystal forms. Below −189 ° C there is crystal form II, above this temperature there is crystal form I. The enthalpy of transformation of the solid phase transition is 0.0441 kJ · mol −1 . The crystal form I melts at 95 ° C with a melting enthalpy of 23.556 kJ mol −1 . The compound boils under a reduced pressure of 16 mbar at 188 ° C. The vapor pressure function results according to Antoine according to log 10 (P) = A− (B / (T + C)) (P in bar, T in K) with A = 4.79729, B = 2780.085 and C = −39.436 in the temperature range from 401.6 to 620 K.
Chemical properties
Benzil is the starting material for the benzilic acid rearrangement .
use
Benzil is used as an intermediate in organic synthesis and as a photoinitiator for certain polymer reactions.
safety instructions
Benzil is irritating to the eyes , the skin and the respiratory tract. The irritation caused can lead to inflammation. The vapors are slightly toxic and should not be inhaled.
Individual evidence
- ↑ a b c d e f g Entry for CAS no. 134-81-6 in the GESTIS substance database of the IFA , accessed on January 5, 2013(JavaScript required) .
- ^ Y. Ogata, Y. Sawaki, T. Ohno: Mechanism for oxidation of phenylacetylenes with peroxymonophosphoric acid. Oxirene as an intermediate inconvertible to ketocarbene. In: J. Am. Chem. Soc. 104, 1982, pp. 216-219, doi: 10.1021 / ja00365a039 .
- ↑ a b A. Dworkin: Heat capacity, phase transition, and thermodynamic properties of benzil. In: J. Chem. Thermodyn. 15, 1983, pp. 1029-1035.
- ↑ a b A. Dworkin, AH Fuchs: Heat capacity of benzil near its phase transition. In: J. Chem. Phys. 67, 1977, pp. 1789-1790.
- ↑ RJL Andon, JE Connett: Calibrants for thermal analysis. Measurement of their enthalpies of fusion by adiabatic calorimetry. In: Thermochim. Acta . 42, 1980, pp. 241-247.
- ^ J. Buckingham, SM Donaghy: Dictionary of Organic Compounds. 5th edition. Chapman and Hall, New York 1982, 1.
- ^ DR Stull: Vapor Pressure of Pure Substances Organic Compounds. In: Ind. Eng. Chem. 39, 1947, pp. 517-540.

