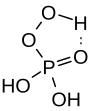
Peroxomonophosphoric acid
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||
| General | |||||||||||||
| Surname | Peroxomonophosphoric acid | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | H 3 PO 5 | ||||||||||||
| Brief description |
colorless, viscous liquid |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 114.00 g mol −1 | ||||||||||||
| Physical state |
liquid |
||||||||||||
| solubility |
soluble in acetonitrile, dioxane |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Peroxomonophosphoric acid is an oxo acid of phosphorus . Their salts are called peroxomonophosphates . In addition to peroxodiphosphoric acid, it is one of the two known peroxophosphoric acids.
history
The two peroxophosphoric acids were first produced and described in 1910 by J. Schmidlin and P. Massini. However, the reaction between phosphorus pentoxide and highly concentrated hydrogen peroxide is very violent and difficult to control. In addition to the use of phosphorus pentoxide, preparations from metaphosphoric acid and diphosphoric acid are also mentioned.
A method of production from phosphorus pentoxide in inert solvents such as diethyl ether , isoamyl alcohol and acetonitrile was described by G. Toennies in 1937, although only the acetonitrile variant was found to be suitable.
Extraction and presentation
Peroxomonophosphoric acid is produced by reacting phosphorus pentoxide with highly concentrated hydrogen peroxide in an inert solvent such as acetonitrile or carbon tetrachloride .
When mixed with peroxodiphosphoric acid, it is also formed when phosphoric acid is treated with fluorine . The connection is not commercially available and must be established prior to use.
properties
Peroxomonophosphoric acid is a colorless, viscous liquid. Stabilization takes place via the formation of an intramolecular hydrogen bridge. The compound is a tri-basic acid. The three acid constants are pK S1 = 1.1, pK S2 = 5.5 and pK S3 = 12.8. A slow hydrolysis to hydrogen peroxide and phosphoric acid takes place in aqueous solution .
In the excess of water, hydrolysis can be viewed as a pseudo-first order reaction. The half-life of the decomposition depends on the pH value and the temperature. They are about 31 hours at 35 ° C and 2.5 hours at 61 ° C. The solution in acetonitrile also decomposes slowly. When stored at 5 ° C, 30% degradation occurs in 26 days. By neutralization with bases such as potassium hydroxide , relatively stable salts such as the hygroscopic potassium dihydrogen peroxomonophosphate KH 2 PO 5 can be obtained.
use
In organic synthesis, peroxomonophosphoric acid is used as an electrophilic reagent for oxidation reactions on alkenes , alkynes , aromatics and amines . The production of relatively acid-stable epoxides can be made from alkenes. Thus, reaction with trans - stilbene the trans -Stilbenoxid. Epoxidation does not work with alkenes such as cyclohexene , styrene or α-methylstyrene . The resulting epoxides are split immediately because of the acid strength of the peroxomonophosphoric acid or the phosphoric acid formed. The reaction with styrene gives phenylacetic acid or with α-methylstyrene 2-phenylpropionic acid.
The oxidation of diphenylethine leads to benzil at room temperature . Oxirene structures are discussed here as intermediates.
Peroxomonophosphoric acid is an effective reagent for the hydroxylation of aromatics. The conversion of mesitylene to mesitol can be achieved within 4 hours at room temperature.
The compound can be used as an effective oxidizing agent in Baeyer-Villiger oxidations . Substituted acetophenones can be converted into the corresponding phenyl acetates in high yield at 30 ° C. The reaction rate is about 100 times higher than when using perbenzoic acid .
Amines such as N , N -dimethylaniline are oxidized to the corresponding N -oxides .
Individual evidence
- ↑ a b c d e H. Jakob, S. Leininger, T. Lehmann, S. Jacobi, S. Gutewort: Inorganic Peroxo Compounds. In: Ullmann's Encyclopedia of Technical Chemistry . Wiley-VCH Verlag, Weinheim 2007, doi : 10.1002 / 14356007.a19_177.pub2 .
- ↑ a b c d e f Monoperoxyphosphoric Acid. In: e-EROS Encyclopedia of Reagents for Organic Synthesis . John Wiley and Sons, 1999-2013, accessed September 17, 2015.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Entry on peroxophosphates. In: Römpp Online . Georg Thieme Verlag, accessed on September 11, 2015.
- ↑ J. Schmidlin, P. Massini: Phosphormonopersäure und überphosphorsäure. In: Chem. Ber. 43, 1910, pp. 1162-1171, doi: 10.1002 / cber.191004301195 .
- ^ G. Toennies: A New Method for the Preparation of Permonophosphoric Acid. In: J. Am. Chem. Soc. 59, 1937, pp. 555-557.
- ↑ T. Zhu, H. Chang, JF Kadla: A new method for the preparation of peroxymonophosphoric acid. In: Can. J. Chem. 81, 2003, pp. 156-160, doi: 10.1139 / v03-010 .
- ↑ a b c C. J. Battaglia, JO Edwards: The Dissociation Constants and the Kinetics of Hydrolysis of Peroxymonophosphoric Acid. In: Inorg. Chem. 4, 1965, pp. 552-558, doi: 10.1021 / ic50026a024 .
- ^ Y. Ogata, K. Tomizawa, T. Ikeda: Oxidation of trans-stilbene with peroxymonophosphoric acid. In: J. Org. Chem. 44, 1979, pp. 2362-2364, doi: 10.1021 / jo01328a006 .
- ^ Y. Ogata, Y. Sawaki, T. Ohno: Mechanism for oxidation of phenylacetylenes with peroxymonophosphoric acid. Oxirene as an intermediate inconvertible to ketocarbene. In: J. Am. Chem. Soc. 104, 1982, pp. 216-219, doi: 10.1021 / ja00365a039 .
- ^ Y. Ogata, Y. Sawaki, K. Tomizawa, T. Ohno: Aromatic hydroxylation with peroxymonophosphoric acid. In: Tetrahedron . 37, 1981, pp. 1485-1486, doi: 10.1016 / S0040-4020 (01) 92087-3 .
- ^ Y. Ogata, K. Tomizawa, T. Ikeda: Kinetics of the Baeyer-Villiger reaction of acetophenones with permonophosphoric acid. In: J. Org. Chem. 43, 1978, pp. 2417-2419, doi: 10.1021 / jo00406a025 .
- ↑ Y. Ogata, Y. Sawaki, T. Morikawa: Kinetics of the peroxymonophosphoric acid oxidation of aromatic amines. In: J. Org. Chem. 44, 1979, pp. 352-355, doi: 10.1021 / jo01317a009 .