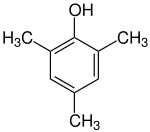
Mesitol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Mesitol | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 9 H 12 O | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 136.19 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
70-72 ° C |
|||||||||||||||
| boiling point |
220 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Mesitol ( 2,4,6-trimethylphenol ) is an aromatic compound with three methyl groups and one hydroxy group . The name and structure of mesitol is derived from the combination of the aromatic compounds mesitylene (symmetrical trimethylbenzene) and phenol .
presentation
Mesitol can be obtained within 4 hours by reacting mesitylene with peroxomonophosphoric acid at room temperature.
Individual evidence
- ↑ a b c d data sheet Mesitol from Sigma-Aldrich , accessed on June 30, 2016 ( PDF ).
- ↑ Ogata, Y .; Sawaki, Y .; Tomizawa, K .; Ohno, T .: Aromatic hydroxylation with peroxymonophosphoric acid , in: Tetrahedron , 1981 , 37 , pp. 1485-1486, doi : 10.1016 / S0040-4020 (01) 92087-3 .


