Cyclobutane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
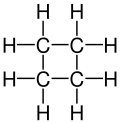
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Cyclobutane | |||||||||||||||
| Molecular formula | C 4 H 8 | |||||||||||||||
| Brief description |
colorless, sweet-smelling gas |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 56.11 g · mol -1 | |||||||||||||||
| Physical state |
gaseous |
|||||||||||||||
| density |
0.71 g cm −3 (11 ° C) |
|||||||||||||||
| Melting point |
−90.73 ° C |
|||||||||||||||
| boiling point |
12.51 ° C |
|||||||||||||||
| Vapor pressure |
130 k Pa (20 ° C) |
|||||||||||||||
| solubility |
very heavy in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
3.7 kJ / mol |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Cyclobutane is a colorless gas belonging to the cycloalkanes with the empirical formula C 4 H 8 .
The cyclobutane molecule
The cyclobutane molecule is not flat, it is in a folded conformation. This reduces the torsional stress ( Pitzer stress ) that would arise in a planar conformation of the molecule through the eight ecliptic hydrogen atoms. According to various measurements, the "kink angle" is 33–37 °.
The non-rigid cyclobutane molecule performs a pseudorotation , with the carbon atoms alternately moving 26 ° out of the plane.
Properties and dangers
Cyclobutane is only slightly soluble in water.
Cyclobutane is extremely flammable.
Reactions
The reactivity of cyclobutane is higher than that of n -butane due to the ring strain . At 500 ° C it is cracked to ethene (reversible - thermally allowed - [2 + 2] cycloaddition ). With hydrogen it is hydrogenated to n- butane over a palladium catalyst . With oxygen , it burns to form water and carbon dioxide .
Web links
- Structure of cyclobutane
- Saša Peter Jacob: Modern radical chemistry in cyclobutane synthesis and in ligand design. Dissertation Rheinische Friedrich-Wilhelms-Universität Bonn 2007, urn : nbn: de: hbz: 5N-12433 .
Individual evidence
- ↑ a b c d e f g Entry on cyclobutane in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-26.


