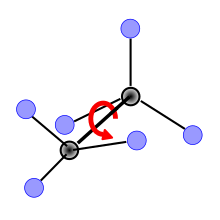
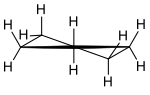
Pseudo rotation
Pseudorotation is a phenomenon that occurs in stereochemistry and describes the rapid change in the positions of atoms or atomic assemblies in molecules and complex compounds . The pseudorotation briefly creates conformational isomers of a compound, i.e. the spatial arrangement of the atoms to one another changes without the chemical bonds between the atoms being broken or newly formed. Pseudorotations occur so quickly that measuring devices such as NMR spectrometers cannot record the change in atomic positions and can only reproduce a structure of the compound averaged over time. The pseudorotation is one of the so-calledintramolecular processes .
The term was coined in 1947 by the US physical chemists John E. Kilpatrick, Kenneth S. Pitzer and Ralph Spitzer, who presented a study of the thermodynamics and molecular structure of the hydrocarbon cyclopentane .
Rotation and pseudo-rotation
Before the development of quantum mechanical theories on chemical bonding and molecular structure, the three-dimensional structure of organic molecules was illustrated using models. Single bonds were represented by rods, the atoms by spheres, which were connected to the rods. (Today's models use metal or plastic building blocks instead of spheres in the case of carbon atoms, which specify the ideal valence angles - 109 ° 28 '(tetrahedral), 120 ° (trigonal), 180 ° (diagonal).)
The ball-and-stick models could lead to the view that parts of a molecule could be rotated “freely” around CC single bonds (“free rotatability”). The simplest example was the hydrocarbon ethane : the two methyl groups can be rotated against each other in the model.
The term rotation is clear because it is based on a simple model, but from a mathematical and physical point of view, oscillations change the internal coordinates of the atoms that make up the molecule.
However, it turned out that for the rotation in the ethane molecule energy has to be expended and an (energy) barrier has to be overcome. Compared to the binding energy of the CC bond, the energy required for rotation is very small.
In the case of smaller cycloalkanes, as can be seen from the ball-and-stick models, a rotation around CC single bonds is not possible without breaking this bond, i.e. H. to supply at least the amount of binding energy. But vibrations, i.e. H. Changes to the internal atomic coordinates are still possible.
The studies on cyclopentane led Pitzer and coworkers to the view that the molecule "folds" or "puckering" (English: puckering ). So one of the five carbon atoms can swing out of the imaginary plane of a regular pentagon; A kind of wave motion runs through the molecule. The researchers called this process "pseudorotation".
Another mode of the pseudo-rotation results from the fact that to two CH 2 groups relative to the other twist (English: twist ). The atomic association shows C 2 symmetry at all times , i.e. H. a twofold axis of rotation. This process is more difficult to visualize.
The molecules of cycloalkanes with ring sizes> 5 also show pseudorotation, but the relationships are more complicated and not easy to show in simple pictures.
Pitzer also recognized what the pseudorotation of cyclopentane and the barrier of rotation of ethane are based on: Nature avoids angles of rotation ( torsion angle ) of zero degrees, so-called “ecliptic interactions” of neighboring ( vicinal ) CH bonds that are unfavorable in terms of energy . Molecules with ecliptic interactions of CH (and also CC) bonds are under " torsional stress ", which is also called "Pitzer stress" after the discoverer. In the case of cyclopentane - if the five carbon atoms were arranged in one plane - all neighboring CH bonds would form an angle of 0 °, i.e. be in an ecliptic position. This would be the maximum of the potential energy; an energetically more favorable arrangement is achieved through pseudo-rotation. The most favorable arrangement are two conformations, which have mirror symmetry (C s ) and C 2 symmetry (twofold axis of rotation): envelope and half-chair shape.
For pseudorotations of other cyclic organic compounds, see the articles on the individual hydrocarbons.
Acyclic compounds from (inorganic) chemistry
The best known and energetically most favorable pseudorotation mechanism was described by the American chemist R. Stephen Berry ( Berry pseudorotation ) for molecules with a trigonal-bipyramidal structure using the example of phosphorus pentafluoride PF 5 .
Berry pseudorotation
Trigonal-bipyramidal molecules using the example of PF 5
The Berry pseudorotation takes place in molecules with a trigonal-bipyramidal structure, the central atom has a coordination number of five. While with coordination numbers of four ( tetrahedron ) or six ( octahedron ) all atoms can have the same distance to the central particle, there are two different distances in a trigonal bipyramid. Three of the five atoms lie in the triangular base of the bipyramid ( equatorial position) and have a shorter distance to the central atom than the two atoms at the tips of the bipyramid ( axial or apical position). In molecules of the form AX 5 , such as the PF 5 shown on the right, three atoms of type X should therefore be distinguishable from the other two; the coordination number five can be described more precisely as 3 + 2. As early as 1953, the American chemist Herbert S. Gutowsky discovered while investigating PF 5 with the help of 19 F - NMR spectroscopy that instead of the expected two signals for the fluorine atoms, only one signal can be observed in the spectra.
Mechanism of the Berry pseudorotation
The atoms in a molecule are not completely rigid in a certain position, but instead vibrate depending on the ambient temperature . These so-called deformation vibrations lead to periodic changes in bond lengths and bond angles . As a result of these deformation vibrations, the two atoms in the Berry pseudorotation move towards each other in the apical position (in the example below No. 4 and 5), the bond angle 4 – central atom – 5 is reduced from 180 ° to 120 ° and the bond lengths are shortened , while two equatorial atoms move away from each other, the bond angle is widened here from 120 ° to 180 ° and the distance to the central particle increases (in the example below No. 1 and 2). The remaining atom in the equatorial position (in example no. 3) is the pivot point (fixed point), the other four atoms move relative to its position. During the change of positions, a transition state is reached in which the four moving atoms form the base of a square pyramid with the pivot point as the pyramid tip. At the end of the pseudorotation, the basic state follows again in the form of a trigonal bipyramid, whereby the formerly apical atoms are now in an equatorial position and vice versa. Since each atom in an equatorial position can serve as a pivot point for the pseudorotation, all atoms occupy both equatorial and apical positions one after the other. The energy requirement for the transition to the square-pyramidal state is around 15 kJ / mol for the Berry pseudorotation for PF 5 .

Since the pseudorotation runs very quickly (pseudorotation frequencies under standard conditions approx. 10 5 s −1 for PF 5 and approx. 10 2 s −1 for phosphorus pentachloride PCl 5 ), it cannot be resolved by an NMR spectrometer and only a time-averaged structure with only one signal for every five atoms can be observed in the spectra. This makes the five atoms appear as if they were similarly connected to the central atom, although in reality they are arranged in a rapidly fluctuating trigonal bipyramid.
The Berry pseudorotation can be observed in some molecules and complexes with a trigonal-bipyramidal structure. In addition to PF 5 and PCl 5 , these also include Fe (PF 3 ) 5 , Fe (CO) 5 and SbMe 5 .
"Inverse Berry pseudorotation"
A kind of “inverse Berry pseudorotation” can be observed in molecules that are basically built up as square pyramids. In the case of iodine pentafluoride IF 5, for example, a pair of fluorine atoms oscillates back and forth in the manner of an opening and closing pair of scissors, causing the entire molecule to vibrate. Because of this oscillation caused by the pendulum movement, all fluorine atoms move with it, in contrast to the normal Berry pseudorotation, and a trigonal bipyramid emerges from the square pyramid for a short time. In this "inverse Berry pseudorotation" the ground and transition states are thus interchanged. However, in addition to the Berry mechanism, this pseudorotation also includes movement patterns of the lever and turnstile mechanisms described below and therefore cannot be viewed as a pure reversal of the Berry pseudorotation.
Further pseudorotation mechanisms
In addition to the Berry pseudorotation, there are other mechanisms that explain possible changes in atomic positions in molecules. The Lever mechanism ( engl. Lever = "lever") describes a possible pseudo rotation of fluorine atoms in sulfur tetrafluoride SF 4 and chlorine trifluoride ClF 3 . Both molecules also have a trigonal-bipyramidal structure, taking into account lone pairs of electrons , whereby in the case of SF 4 an equatorial position is occupied by the lone pair of electrons from sulfur , in ClF 3 the two lone pairs of electrons from chlorine occupy equatorial positions (see also VSEPR -Model ). The pseudorotation is caused by a fluorine atom, which performs a movement similar to a lever that is pushed back and forth, and thereby also sets the remaining fluorine atoms in vibration. The pseudorotation is carried out exclusively by the fluorine atoms, since lone pairs of electrons always prefer equatorial positions due to their increased space requirement. In the case of SF 4 , a Berry pseudorotation can also take place with the lone pair of electrons as the pivot. The mean structure for SF 4, without taking the lone pair of electrons into account, is the shape of a disphenoid (an elongated tetrahedron ). The energy requirement for executing the lever mechanism is approx. 42 kJ / mol higher than for the Berry pseudorotation. In the case of ClF 3 , only the lever mechanism is possible due to the two free electron pairs. The T-shaped molecule (without taking the lone pair of electrons into account) takes on a trigonal-planar shape as a transitional shape.
For molecules with an octahedral structure, there are calculations on the energy requirement for changing chlorine atoms into cis-trans isomers of the hypothetical compound sulfur dichloride tetrafluoride SCl 2 F 4 . The change of the chlorine atoms from a cis to a trans arrangement would require approx. 272 kJ / mol. The movement would be caused by two fluorine atoms counterclockwise with simultaneous rotation of the other two clockwise. This type of pseudorotation is known as the turnstile mechanism .
The Bartell mechanism is a pseudorotation similar to the Berry mechanism and was first described on iodine heptafluoride IF 7 with a pentagonal-pyramidal molecular shape. Here, too, there is a paired exchange of apical and equatorial fluorine atoms in the pentagonal bipyramid. The mechanism has characteristics of the Berry, Lever and Turnstile pseudorotation.
Literature and Sources
- M. Binnewies, M. Jäckel, H. Willner, G. Rayner-Canham: General and Inorganic Chemistry . 1st edition, Spektrum, Heidelberg 2004, ISBN 978-3-82-740208-0 , p. 488.
- ME Cass, KK Hii, HS Rzepa: Mechanisms that Interchange Axial and Equatorial Atoms in Fluxional processes: Illustration of the Berry Pseudorotation, the Turnstile and the Lever Mechanisms via animation of transition state normal vibrational modes. In: Journal of Chemical Education (Online) . Vol. 83, No. 2, 2006, p. 336.
- AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 782.
- RR Holmes: Structure of Cyclic Pentacoordinated Molecules of Main Group Elements . In: Phosphorus, Sulfur, and Silicon and the Related Elements . Vol. 98, No. 1-4, 1995, pp. 205-221.
- JE Huheey, E. Keiter, RL Keiter: Inorganic Chemistry. Principles of structure and reactivity . 3rd edition, de Gruyter, Berlin 2004, ISBN 978-3-11-017903-3 , pp. 275 ff.
- R. Luckenbach: Dynamic Stereochemistry of Pentacoordinated Phosphorus and Related Elements . 1st edition, Thieme, Stuttgart 1973, ISBN 3-13-456801-2 .
- I. Ugi, D. Marquarding, H. Klusacek, P. Gillespie: Berry Pseudorotation and Turnstile Rotation . In: Accounts of Chemical Research . Vol. 4, No. 8, 1971, pp. 288-296.
Individual evidence
- ^ John E. Kilpatrick, Kenneth S. Pitzer, Ralph Spitzer: The Thermodynamics and Molecular Structure of Cyclopentane, Journal of the American Chemical Society 69, 2483-2488 (1947). doi : 10.1021 / ja01202a069 .
- ↑ RS Berry: Correlation of Rates of Intramolecular Tunneling Processes, with Application to Some Group V Compounds . In: Journal of Chemical Physics . Vol. 32, No. 3, 1960, 32, pp. 933-938.
- ^ HS Gutowsky, DW McCall, CP Slichter: Nuclear Magnetic Resonance Multiplets in Liquids . In: Journal of Chemical Physics . Vol. 21, No. 2, 1953, pp. 279-292.
- ↑ a b c M. E. Cass, KK Hii, HS Rzepa: Mechanisms that Interchange Axial and Equatorial Atoms in Fluxional processes: Illustration of the Berry Pseudorotation, the Turnstile and the Lever Mechanisms via animation of transition state normal vibrational modes. In: Journal of Chemical Education (Online) . Vol. 83, No. 2, 2006, p. 336.
- ^ WJ Adams, H. Bradford Thompson, LS Bartell: Structure, Pseudorotation, and Vibrational Mode Coupling in IF 7 : An Electron Diffraction Study . In: Journal of Chemical Physics . Vol. 53, No. 10, 1970, pp. 4040-4046.