Platonic hydrocarbons
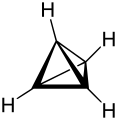
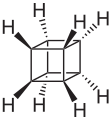
As Platonic hydrocarbons is known that saturated hydrocarbons , the carbon skeletons, the geometric structures Platonic solid own. These are the compounds tetrahedrane ( tetrahedron ), cubane ( cube ), octahedrane ( octahedron ), dodecahedrane ( dodecahedron ) and icosahedrane ( icosahedron ).

For orbital symmetry reasons, however, octahedrane and icosahedrane are only of theoretical character and potentially cannot be represented as real molecules. In the case of Octahedrans the four would have bonds of the skeleton carbon atoms in the direction of the corner points of the square plane of the octahedron demonstrate what such a strong deviation from the tetrahedral angle of the sp 3 hybrid orbitals would mean the carbon atoms that no bond formation is possible. In Icosahedran the structure would need to train the additional four-bonded carbon atoms form a fifth bond.
Tetrahedrane could not yet be synthesized due to its high ring tension , but its tetrakis ( tert- butyl) derivative could be produced in 1978. The bulging tert-butyl radicals stabilize the strained structure of the tetrahedron skeleton . The tension energies of the three known compounds decrease from the tetrahedrane via the cubane to the dodecahedrane.
Several routes are known for the synthesis of cubane and dodecahedrane. Cubane was first synthesized in 1964 and dodecahedrane was first synthesized in 1982.
Individual evidence
- ↑ G. Maier, S. Pfriem, U. Schäfer, R. Matusch: Tetra-tert-butyltetrahedrane , in: Angew. Chem. Int. Ed. , 1978 , 17 , pp. 520-521; doi: 10.1002 / anie.197805201 .
- ^ PE Eaton, TW Cole: Cubane , in: J. Am. Chem. Soc. , 1964 , 86 , pp. 3157-3158; doi: 10.1021 / ja01069a041 .
- ^ PE Eaton, TW Cole: The Cubane System , in: J. Am. Chem. Soc. , 1964 , 86 , pp. 962-964; doi: 10.1021 / ja01059a072 .
- ↑ LA Paquette, MJ Wyvratt: Domino Diels-Alder Reactions. I. Applications to the Rapid Construction of Polyfused Cyclopentanoid Systems , in: J. Am. Chem. Soc. , 1974 , 96 , pp. 4671-4673; doi: 10.1021 / ja00821a052 .
- ↑ LA Paquette, MJ Wyvratt, O. Schallner, JL Muthard, WJ Begley, RM Blankenship, D. Balogh: Topologically Spherical Molecules. Synthesis of a Pair of C 2 -Symmetric Hexaquinane Dilactones and Insights into Their Chemical Reactivity. An Efficient π-Mediated 1,6-Sicarbonyl Reduction , in: J. Org. Chem. , 1979 , 44 , pp. 3616-3630; doi: 10.1021 / jo01335a003 .
- ↑ W.-D. Fessner, BARC Murty, H. Prinzbach : The Pagodane Route to Dodecahedranes - Thermal, Reductive, and Oxidative Transformations of Pagodanes , in: Angew. Chem. Int. Ed. , 1987 , 26 , pp. 451-452; doi: 10.1002 / anie.198704511 .
- ↑ W.-D. Fessner, BARC Murty, J. Wörth, D. Hunkler, H. Fritz, H. Prinzbach, WD Roth, P. von Ragué Schleyer, AB McEwen, WF Maier: Dodecahedranes from [1.1.1.1] Pagodanes , in: Angew. Chem. Int Ed. , 1987 , 26 , pp. 452-454 doi: 10.1002 / anie.198704521 .
literature
- P. Ball, A. Schleitzer: Platonic Molecules , in: Brillante Denker, Kühne Pioneers: Ten groundbreaking discoveries , Wiley-VCH, Weinheim, 1st edition, 2007 , pp. 197-213, ISBN 3-527-31680-9 .
- W. Grahn: Platonic hydrocarbons in: Chemistry in our time , 1981 , 15 , pp. 52-61; doi: 10.1002 / ciuz.19810150205 .