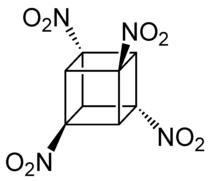
Tetranitrocubane
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| General | ||||||||||
| Surname | Tetranitrocubane | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 8 H 4 (NO 2 ) 4 | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 284 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| density |
1.83 g cm −3 |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Tetranitrocubane (TNC for short) is a chemical compound with the molecular formula (C 8 H 4 (NO 2 ) 4 ) that can be used as an explosive .
The cubane (C 8 H 8 ) basic structure has four nitro groups and four hydrogen atoms, whereas octanitrocubane has eight nitro groups and no more hydrogen atoms . Tetranitrocubane is produced on a kilometer scale because a potential explosive has been recognized in this molecule .
The high-energy structure of the cube geometry increases the explosiveness of the explosives.
swell
- ↑ Jinshan Li: An evaluation of nitro derivatives of cubane using ab initio and density functional theories in Theor. Chem. Account. 122 (2009) 101-106, doi : 10.1007 / s00214-008-0489-5 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
literature
- George A. Olah (Eds), David R. Squire: Chemistry of Energetic Materials . Academic Press 1991; ISBN 0125254407