2-cyclohexen-1-one
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 2-cyclohexen-1-one | ||||||||||||||||||
| other names |
Cyclohex-2-enone |
||||||||||||||||||
| Molecular formula | C 6 H 8 O | ||||||||||||||||||
| Brief description |
colorless liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 96.13 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.99 g cm −3 (20 ° C) |
||||||||||||||||||
| Melting point |
−53 ° C |
||||||||||||||||||
| boiling point |
167-169 ° C |
||||||||||||||||||
| Vapor pressure |
1011 h Pa (168 ° C) |
||||||||||||||||||
| solubility |
soluble in water |
||||||||||||||||||
| Refractive index |
1.4883 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
2-Cyclohexen-1-one is a chemical compound from the group of enones , i. H. a ketone with a C = C double bond . In its pure state the substance is a colorless liquid; the commercially available product is mostly yellow in color.
Extraction and presentation
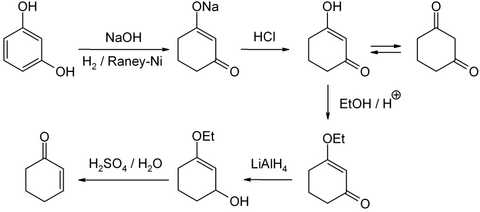
There are several different synthetic routes for the preparation of 2-cyclohexen-1-one, of which only a few are mentioned here:
A well-developed method for the laboratory scale is the reduction and acidic hydrolysis of 3-ethoxy-2-cyclohexen-1-one, which in turn is accessible from resorcinol via 1,3-cyclohexanedione:
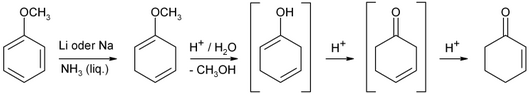
If liquid ammonia is available, it can be obtained from anisole by Birch reduction with subsequent acidic hydrolysis and rearrangement of the C – C double bond:
It can also be obtained quite easily from cyclohexanone by α-bromination and elimination , or from 3-chlorocyclohexene by hydrolysis and oxidation .
Technically, 2-cyclohexen-1-one is produced by catalytic oxidation of cyclohexene , e.g. B. with hydrogen peroxide on vanadium catalysts. Several process variants with different oxidizing agents or catalysts are patented.
properties
Physical Properties
2-Cyclohexen-1-one has a melting point of −53 ° C, a boiling point of around 170 ° C and a flash point of 56 ° C. At room temperature it is an easily mobile, clear liquid with a density of 0.993 g · cm −3, similar to that of water.
It is compatible with many solvents, such as B. alcohols ( methanol , ethanol ), ethers ( diethyl ether , tetrahydrofuran , 1,4-dioxane , tert-butyl methyl ether), haloalkanes ( dichloromethane , chloroform ), esters ( ethyl acetate ) and also with polar, aprotic solvents ( dimethylformamide , dimethyl sulfoxide ) unlimited miscible.
The solubility in water is 41.3 g · l −1 at pH 7 and 25 ° C.
Chemical properties
2-Cyclohexen-1-one can enter into both the usual reactions of ketones (e.g. acetal formation ) and of alkenes (e.g. electrophilic additions , cycloadditions , epoxidation ). As a typical representative of the α, β-unsaturated carbonyl compounds , it has an electron-poor C – C double bond, which can also function as an electrophile . With strong bases it can be deprotonated at positions 4 and 6 (the two CH 2 groups that are adjacent to the carbonyl group or the C – C double bond) .
use
It is an often used synthetic building block in organic chemistry, as it offers many different possibilities for expanding the molecular structure. For example, it can easily be converted in a Michael addition with nucleophiles (such as enolates or silyl enol ethers) or in the sense of a Diels-Alder reaction with electron-rich dienes . Furthermore, it reacts with organocopper compounds under 1,4-addition (Michael addition) or with Grignard compounds under 1,2-addition, i. H. with attack of the nucleophile on the carbonyl carbon atom. It is z. B. used in multi-stage total syntheses in the construction of polycyclic natural products .
literature
- WF Gannon, HO House: 2-Cyclohexenones In: Organic Syntheses . 40, 1960, p. 14, doi : 10.15227 / orgsyn.040.0014 ; Coll. Vol. 5, 1973, p. 294 ( PDF ).
- WF Gannon, HO House: 3-Ethoxy-2-Cyclohexenone In: Organic Syntheses . 40, 1960, p. 41, doi : 10.15227 / orgsyn.040.0041 ; Coll. Vol. 5, 1973, p. 539 ( PDF ).
- RB Thompson: Dihydroresorcinol In: Organic Syntheses . 27, 1947, p. 21, doi : 10.15227 / orgsyn.027.0021 ; Coll. Vol. 3, 1955, p. 278 ( PDF ).
Individual evidence
- ↑ a b c d e f data sheet 2-Cyclohexen-1-one at AlfaAesar, accessed on December 15, 2010 ( PDF )(JavaScript required) .
- ↑ Data sheet 2-Cyclohexen-1-one (PDF) from Merck , accessed on March 20, 2011.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-128.
- ↑ a b c Entry on 2-cyclohexen-1-one in the GESTIS substance database of the IFA , accessed on January 15, 2020(JavaScript required) .