Anisole
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
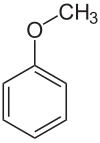
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Anisole | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 7 H 8 O | ||||||||||||||||||
| Brief description |
colorless liquid with a pleasant odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 108.14 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.99 g cm −3 (20 ° C) |
||||||||||||||||||
| Melting point |
−37 ° C |
||||||||||||||||||
| boiling point |
154 ° C |
||||||||||||||||||
| Vapor pressure |
3.6 hPa (20 ° C) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| Dipole moment | |||||||||||||||||||
| Refractive index |
1.516 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Anisole is a characteristically smelling, colorless, flammable liquid. Since anisole can formally be understood as an ether made from phenol and methanol , it is also referred to as methylphenyl ether or methoxybenzene .
presentation
On a laboratory scale, anisole can be obtained by etherification of phenol or phenolates ( ether synthesis according to Williamson ), e.g. B. by reacting a phenolate with methyl iodide or phenol with dimethyl sulfate in the presence of a base .
use
Anisole is used as a solvent , a heat transfer medium (between 150 and 260 ° C) and a raw material for the synthesis of organic compounds such as drugs and fragrances .
Related structures
Name and structurally derived from Anis three other compounds ol by the introduction of a carbon atom from: anise alcohol, anise aldehyde, anise acid. The representation of anisaldehyde by means of Vilsmeier formylation .
-CH 2 OH –CHO -COOH 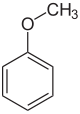

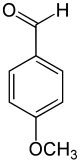

Anisole Anise alcohol Anisaldehyde Anisic acid
The derivatives also include:
- Anisolsulfonic acids
- Phenetol (ethoxybenzene)
- Anisidine (Aminoanisole)
- Methyl anisoles
- Nitro anisole
- Thioanisole (phenol thioether)
Individual evidence
- ↑ a b c d e f g h i Entry on anisole in the GESTIS substance database of the IFA , accessed on March 17, 2017(JavaScript required) .
- ↑ Entry on anisole. In: Römpp Online . Georg Thieme Verlag, accessed on March 10, 2014.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Dipole Moments, pp. 9-52.
- ↑ Anisole data sheet from Sigma-Aldrich , accessed on June 25, 2011 ( PDF ).
- ^ Association of authors: Organikum , 19th edition, Johann Ambrosius Barth, Leipzig · Berlin · Heidelberg 1993, ISBN 3-335-00343-8 , p. 345.



