Anisolsulfonic acids
| Anisolsulfonic acids | ||||||
| Surname | 2-anisolesulfonic acid | 3-anisolesulfonic acid | 4-anisolesulfonic acid | |||
| other names |
o -anisolsulfonic acid 2-methoxybenzenesulfonic acid |
m -anisolsulfonic acid 3-methoxybenzenesulfonic acid |
p -Anisolsulfonic acid 4-methoxybenzenesulfonic acid |
|||
| Structural formula |
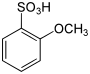
|

|

|
|||
| CAS number | 5857-42-1 | |||||
| PubChem | 12406954 | 12037036 | 604723 | |||
| Molecular formula | C 7 H 8 O 4 S | |||||
| Molar mass | 188.20 g mol −1 | |||||
| Physical state | firmly | |||||
| Brief description | ||||||
| Melting point | ||||||
|
GHS labeling |
|
|
|
|||
| H and P phrases | see above | see above | see above | |||
| see above | see above | see above | ||||
The anisolesulfonic acids form a group of substances in chemistry that is derived from both benzenesulfonic acid and anisole . The structure consists of a benzene ring with attached sulfonic acid (–SO 2 OH) and methoxy group (–OCH 3 ) as substituents . Their different arrangement results in three constitutional isomers with the empirical formula C 7 H 8 O 4 S.
literature
- FLS Purgato, MIC Ferreira, JR Romero: "Electrocatalytic oxidation of alcohols, diols and arenes with ceric p -methoxybenzenesulfonate and ceric p -toluenesulfonate", in: Journal of Molecular Catalysis A: Chemical 2000 , 161 (1-2) , 99– 104; doi : 10.1016 / S1381-1169 (00) 00192-8 .
Web links
- Patent US3931295 : Hydroxylation of aromatic compounds. Applied July 23, 1974 , published January 6, 1976 , Applicant: Universal Oil Products Company, Inventor: Stephen N. Massie.
See also
- 3-Amino-4-anisolsulfonic acid , CAS number: 98-42-0
- 4-Hydroxy-4-anisolsulfonic acid , CAS number: 50855-43-1